US Pharm. 2011;36(12):34-38.
National Influenza Vaccination Week (December 4-10, 2011) was established to highlight the importance of continuing influenza vaccination after the holiday season.1 The nation is well below the immunization goals set by Healthy People 2020 and influenza is still one of the leading causes of illness and death in the United States, accounting for substantial spending on the related consequences of infection.2 This article will highlight the key recommendations of the Advisory Committee on Immunization Practices (ACIP) for the 2011-2012 influenza season and present the new vaccines that have been added to combat this preventable infectious disease.
General Recommendations
The 2011-2012 seasonal influenza vaccine virus strains are identical to those contained in the 2010-2011 vaccine. The influenza A (H1N1) strain is derived from the 2009 pandemic influenza virus and includes A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2), and B/Brisbane/60/2008. Routine annual influenza vaccination is recommended for all individuals aged at least 6 months. Vaccination should optimally occur before the onset of influenza activity in the community and be offered throughout the influenza season. Even though the vaccine virus strains are unchanged from last year, annual vaccination is still necessary since several studies have shown that postvaccination antibody titers decline over the course of a year.3
Influenza vaccination is especially important for individuals who are at increased risk for severe complications from influenza. The most frequent serious complications are pulmonary and fall into four categories: primary influenza pneumonia, secondary bacterial pneumonia, pneumonia due to unusual pathogens in immunocompromised hosts, and exacerbations of chronic pulmonary diseases. The most common bacterial pathogens are Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae, which act synergistically with influenza virus through increased binding and invasion of bacteria, increased viral replication, and modification of the host inflammatory response. As the viral neuraminidase works to release new viral particles from host cells, damage to the epithelial layer of the airway occurs, exposing binding sites necessary for adherence of the bacteria. During the 2003-2004 and 2006-2007 influenza seasons, cases of severe community-acquired pneumonia due to methicillin-resistant S aureus (MRSA) were reported with 33% mortality.4
Data collected during the 2009 pandemic not only established that groups already known to be at increased risk for influenza complications were also at greater risk for novel H1N1-related complications, but also identified new high-risk groups—American Indians/Alaska Natives and morbidly obese individuals.5 Observational studies demonstrated a higher risk for death in these two groups, which may have reflected a higher prevalence of underlying chronic medical conditions. The ACIP still recommends that when vaccine supply is limited, vaccination efforts should first concentrate on delivering influenza vaccine to the high-risk individuals shown in TABLE 1.5
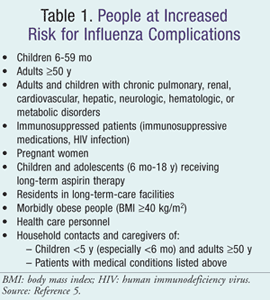
Children
For children, the risk for hospitalization for severe complications from influenza is highest among those aged less than 2 years, and medical care and emergency department visits attributable to influenza are increased among children aged less than 5 years.5 Nearly half of the children who died from influenza virus infections during the 2010-2011 influenza season had no known ACIP-defined high-risk medical condition and were aged less than 5 years.6 Children aged 6 months through 8 years require two doses of influenza vaccine administered at least 4 weeks apart during their first season of vaccination to optimize response. Previously, if a child in this age group received only one dose of influenza vaccine during the prior season, two doses would still need to be given the next season. However, because the 2011-2012 vaccine strains are unchanged from last season, children in this age group who received at least one dose of the 2010-2011 seasonal vaccine will only require one dose of the 2011-2012 vaccine this year. Of course, if the health care provider cannot verify that at least one dose was given in the 2010-11 season, then two doses should be administered at least 4 weeks apart.
The results of three meta-analyses have shown that both trivalent inactivated vaccine (TIV) and live attenuated influenza vaccine (LAIV) reduce laboratory confirmed influenza illness as well as clinical influenza-like illness among children.7 Although effectiveness of the influenza vaccine is well documented, the perception of vaccine safety is the most significant obstacle to overcome in convincing parents to annually vaccinate their young children. A large cohort study of over 66,000 children who received more than 91,000 TIV doses concluded that there was no evidence of serious events following vaccination with TIV among children aged 24 to 59 months.8 Evidence also supports that LAIV is an effective and well-tolerated pediatric vaccine in children 2 to 7 years of age without high-risk underlying medical conditions.9
Elderly
Adults aged 65 years and older are at greater risk for hospitalization and death from seasonal influenza and respond to vaccination with lower antibody titers to influenza hemagglutinin compared with younger adults.10 A 2010 Cochrane Review concluded that available evidence provides no guidance regarding the safety or effectiveness of influenza vaccines for people aged 65 years and older.11 Recent studies have produced varied results regarding vaccine effectiveness. Baxter et al found that over 11 influenza seasons, vaccination prevented 8.5% of hospitalizations for pneumonia and influenza in those 65 years and older, and 12.4% in those 50 to 64 years.12 Talbot et al reported that influenza vaccination prevented 61% of respiratory hospitalizations in community-dwelling older adults 50 years and older.13
Immunogenicity data among persons 65 years and older indicate that higher-dose preparations elicit substantially higher hemagglutinin inhibition titers compared with the standard dose.5 In December 2009, the FDA licensed an injectable TIV indicated for people 65 years and older (Fluzone High-Dose, Sanofi Pasteur, Inc.) that contains four times the standard amount of hemagglutinin antigen per dose, specifically 60 mcg versus the standard 15 mcg of each influenza virus strain.13 Fluzone High-Dose comes in a prefilled syringe and is administered as a single dose of 0.5 mL intramuscularly (IM).14 The most commonly reported adverse reactions are localized injection-site pain, systemic headache, myalgia, and malaise, all of which are typically mild and transient. Because antibody responses of standard Fluzone have been shown to be lower in individuals 60 years and older compared to younger adults, a 3-year postlicensure study started in 2009 is currently assessing the vaccine effectiveness of Fluzone High-Dose compared with standard Fluzone.13,14 Current ACIP recommendations do not express a preference for any specific licensed TIV for use in people 65 years and older.3
Pregnant Women and Infants
Women are at increased risk for morbidity and mortality from influenza during pregnancy, and vaccination can protect both the woman and her infant, especially one aged less than 6 months who is not old enough to receive influenza vaccination.15 The ACIP and the American College of Obstetricians and Gynecologists (ACOG) recommend the administration of TIV for all women who are pregnant during the influenza season, regardless of pregnancy trimester.5,16 An analysis of Vaccine Adverse Event Reporting System (VAERS) reports identified no difference in the safety profile of preservative-containing versus preservative-free TIV vaccines in infants aged 6 to 23 months; therefore, the ACIP advises any age- and risk factor–appropriate vaccine preparation for all persons recommended to receive TIV.5 Although pregnancy is associated with tolerance to foreign antigens and a decrease in total circulating immunoglobulin levels, data from 2009 H1N1 influenza vaccination demonstrated that a single dose of TIV was highly immunogenic in women vaccinated during the second or third trimester of pregnancy.17 Additionally, although antibody titers decreased in the interval between vaccination and delivery, relatively high titers were maintained up to the time of delivery, and cord blood titers tended to be higher than maternal delivery titers.
Infection with the pandemic virus was not only associated with increased maternal death, but also adverse neonatal outcomes. In addition to the protection that is provided to pregnant women, vaccination in pregnancy provides benefits to their infants through transplacental transfer of hemagglutinin inhibition antibodies to the fetus.18 Infants of vaccinated mothers were 45% to 48% less likely to have influenza hospitalizations and had a 41% lower risk of laboratory-confirmed influenza infection than infants of unvaccinated mothers.18,19
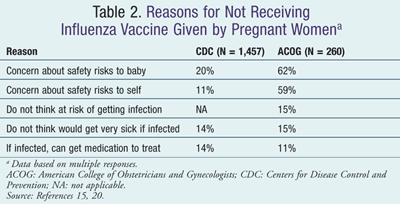
Before 2009, estimated influenza vaccination coverage among pregnant women had been consistently low (about 15%), but levels increased substantially in response to the 2009 influenza A (H1N1) pandemic to nearly 50%. This increased level of vaccination was maintained during the 2010-2011 season (49%), but is still well below the Healthy People 2020 goal of 80%.2,15 Surveys have been conducted to determine why pregnant women do not get vaccinated, and the main reasons are depicted in TABLE 2.15,20 Concern about the safety of influenza vaccination is a persistent barrier; therefore, the Vaccines and Medications in Pregnancy Surveillance System (VAMPSS), coordinated by the American Academy of Allergy, Asthma and Immunology (AAAAI), has been designed to assess systematically the safety of vaccines and medications during pregnancy.20,21 Through a comprehensive assessment of perinatal outcomes, the VAMPSS should allow enhanced prevention and improved treatment of influenza during pregnancy by the identification of any exposures that might be associated with important risks and the provision of reassurance for exposures that are found to be relatively safe.21
Egg Allergy
Several recent studies have documented safe receipt of TIV in people with egg allergy, and recent revisions of some TIV package inserts note that only severe allergic reaction, namely anaphylaxis, to egg protein is a contraindication. The quantity of egg protein in vaccine is expressed as the concentration of ovalbumin per dose, and studies have reported that up to 1.4 mcg/mL has been tolerated without serious reactions.3 Webb et al performed a retrospective review of the safety of seasonal and H1N1 influenza vaccinations in patients with egg allergy during the 2009-2010 season.22 No systemic reactions in any patients, including 23% with a history of severe egg allergy, were witnessed in 285 vaccinations.22 Prospective studies are ongoing to confirm these findings.
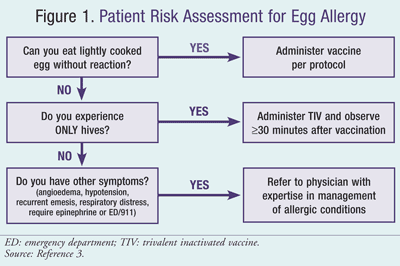
ACIP recommendations still caution that a previous severe allergic reaction to influenza vaccine, regardless of the component suspected, is a contraindication to receipt of influenza vaccine, but further delineation of the severity of egg allergy and appropriate administration of the vaccine have been incorporated into the 2011 recommendations.3 FIGURE 1 illustrates the recommended risk assessment for a patient with egg allergy and the corresponding vaccine protocol. Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs in addition to skin and/or blood testing for immunoglobulin E antibodies to egg proteins. All vaccines should be administered in settings where personnel and equipment for rapid recognition and treatment of anaphylaxis are available.3
Vaccine and Antiviral Agent Updates
Influenza vaccine can now be administered via three different routes: IM TIV, intradermal TIV, and intranasal LAIV, as depicted in TABLE 3. The new IM Fluzone High-Dose TIV has already been discussed in terms of its use in individuals 65 years and older, but in May 2011 Fluzone Intradermal TIV was approved for people aged 18 to 64 years. The intradermal formulation comes in a prefilled microinjection system that delivers 9 mcg of each influenza strain per 0.1 mL dose. Injection-site reactions, namely erythema, induration, swelling, and pruritus, were significantly more frequent with Fluzone Intradermal than IM Fluzone.14
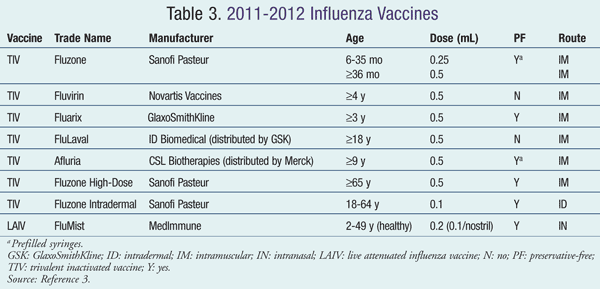
In studies comparing IM Fluzone to Fluzone Intradermal, the intradermal product performed as well as the IM vaccine in all aspects except the seroconversion rate to the influenza B strain.14 Serologic data are not always indicative of how well a vaccine will protect individuals in a specific influenza season, since effective vaccines must be both immunogenic and well matched for circulating virus strains; therefore, the 2011-2012 influenza season will be a good opportunity to assess the clinical effectiveness of Fluzone Intradermal. Additionally, the smaller needle on the intradermal product may serve as a tool for pharmacists to persuade individuals who are afraid of IM vaccination to receive the influenza vaccine.
All IM Fluzone products are rated Pregnancy Category C, whereas Fluzone Intradermal is Pregnancy Category B, since animal studies have resulted in no deleterious effects.14 No studies have been performed in pregnant women for any of the Fluzone products, but the ACIP still recommends annual vaccination of pregnant women with TIV owing to their high risk for influenza complications, and does not favor any specific TIV product.
In 2011, a change in age indication occurred in the Afluria package insert, manufactured by CSL Biotherapies.23 This IM TIV was originally indicated for those 6 months and older, but the identical Australian product (Fluvax Junior and Fluvax) was associated with increased frequency of fever and febrile seizures in children aged 6 months to 4 years, as well as with increased reports of fever in children aged 5 to 8 years.24 The manufacturing process for the 2010-2011 Fluvax Junior and Fluvax was the same as for Afluria, and the vaccine strains were antigenically equivalent, except for influenza A (H3N2). As a result, the ACIP recommended that the 2010-2011 Afluria vaccine not be administered to children aged 6 months to 8 years and that other age-appropriate licensed seasonal influenza vaccine formulations be used in these children. As of 2010, no biological cause has been identified to explain the increase in febrile reactions.24 The minimum age for Afluria is now 5 years, and the following statement has been added to Warnings and Precautions in the package insert: “Administration of CSL’s 2010 Southern Hemisphere influenza vaccine has been associated with increased postmarketing reports of fever and febrile seizures in children predominantly below the age of 5 years as compared to previous years.”23 The ACIP reaffirms its recommendation regarding Afluria for the 2011-2012 season.24
In January 2011, the ACIP issued updated recommendations regarding the use of antiviral agents for the treatment and chemoprophylaxis of influenza. The key points are: 1) early treatment with oseltamivir or zanamivir should be used in people who have severe, complicated, or progressive illness or who require hospitalization or are at higher risk for complications; 2) amantadine and rimantadine should not be used due to high levels of resistance among circulating influenza A viruses; 3) oseltamivir may be used in infants <1 year when indicated; and 4) antiviral treatment based on clinical judgment may be considered for any outpatient with influenza if initiated within 48 hours of illness onset.25
Conclusion
Despite the evidence supporting the effectiveness and safety of influenza vaccines, vaccination rates for all populations fall short of the Healthy People 2020 objectives. An alarming statistic is that only 63.5% of health care personnel (HCP) were vaccinated in the 2010-2011 influenza season, well below the Healthy People 2020 target of 90%.26 Although vaccination of HCP has been shown to reduce illness, absenteeism, and transmission to others, the National Health Interview Survey (NHIS) indicated that 66.2% of unvaccinated HCP believed that the influenza vaccine was safe but thought that getting vaccinated was not worth the time and expense.26 All HCP have an ethical obligation to protect vulnerable patients and do no harm. Hopefully, pharmacists will take the lead in the 2011-2012 season to receive and administer the influenza vaccine to serve as an example for others and move vaccination rates closer to the goals established by Healthy People 2020.
REFERENCES
1. CDC. National Influenza Vaccination Week.
www.cdc.gov/flu/NIVW/. Accessed September 28, 2011.
2. Immunizations and infectious diseases. Healthy People 2020.
www.healthypeople.gov/2020/
3. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1128-1132.
4. Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121:258-264.
5. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1-62.
6. CDC. Influenza-associated pediatric deaths–United States, September 2010-August 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1233-1238.
7. Nichol KL. Efficacy and effectiveness of influenza vaccination. Vaccine. 2008;26(suppl 4):D17-D22.
8. Glanz JM, Newcomer SR, Hambidge SJ, et al. Safety of trivalent inactivated influenza vaccine in children aged 24 to 59 months in the vaccine safety datalink. Arch Pediatr Adolesc Med. 2011;165:749-755.
9. Belshe RB, Ambrose CS, Yi T. Safety and efficacy of live attenuated influenza vaccine in children 2-7 years of age. Vaccine. 2008;26(suppl 4):D10-D16.
10. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ³65 years (Fluzone High-Dose) and guidance for use–United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
11. Jefferson T, Di Pietrantonj C, Al-Ansary LA, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2010;(2):CD004876.
12. Baxter R, Ray GT, Fireman BH. Effect of influenza vaccination on hospitalizations in persons aged 50 years and older. Vaccine. 2010;28:7267-7272.
13. Talbot HK, Griffin MR, Chen Q, et al. Effectiveness of seasonal vaccine in preventing confirmed
influenza-associated hospitalizations in community dwelling older adults. J Infect Dis. 2011;203:500-508.
14. Fluzone, Fluzone High-Dose, and Fluzone Intradermal (influenza virus vaccine) package insert. Swiftwater, PA: Sanofi Pasteur, Inc; 2011.
15. CDC. Influenza vaccination coverage among pregnant women–United States, 2010-11 influenza season. MMWR Morb Mortal Wkly Rep. 2011;60:1078-1082.
16. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 468: influenza vaccination during pregnancy. Obstet Gynecol. 2010;116:1006-1007.
17. Jackson LA, Patel SM, Swamy GK, et al. Immunogenicity of an inactivated monovalent 2009 H1N1 influenza vaccine in pregnant women. J Infect Dis. 2011;204:854-863.
18. Rasmussen SA, Kissin DM, Yeung LF, et al. Preparing for influenza after 2009 H1N1: special considerations for pregnant women and newborns. Am J Obstet Gynecol. 2011;204(suppl 1):S13-S20.
19. Poehling KA, Szilagyi PG, Staat MA, et al. Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol. 2011;204(suppl 1):S141-S148.
20. Steelfisher GK, Blendon RJ, Bekheit MM, et al. Novel pandemic A (H1N1) influenza vaccination among pregnant women: motivators and barriers. Am J Obstet Gynecol. 2011;204(suppl 1):S116-S123.
21. Schatz M, Chambers CD, Jones KL, et al. Safety of influenza immunizations and treatment during pregnancy: the Vaccines and Medications in Pregnancy Surveillance System. Am J Obstet Gynecol. 2011;204(suppl 1):S64-S76.
22. Webb L, Petersen M, Boden S, et al. Single-dose influenza vaccination of patients with egg allergy in a multicenter study. J Allergy Clin Immunol. 2011;128:218-219.
23. Afluria (influenza virus vaccine) package insert. Whitehouse Station, NJ: Merck & Co., Inc; 2011.
24. CDC. Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010-11. MMWR Morb Mortal Wkly Rep. 2010;59:989-992.
25. CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1-18.
26. CDC. Influenza vaccination coverage among health-care personnel–United States, 2010-11 influenza season. MMWR Morb Mortal Wkly Rep. 2011;60:1073-1077.
To comment on this article, contact rdavidson@uspharmacist.com.





