US Pharm. 2007;32(1)(Oncology suppl):7-21.
In
the United States, kidney cancer (renal cell carcinoma) is estimated to be
the seventh most common cancerous condition diagnosed in men and the 13th most
common in women, accounting for 2.7% of all malignant conditions.1
Throughout 2006, an estimated 38,890 new cases of kidney and renal pelvis
cancer will be diagnosed, and approximately 13,000 patients with the disease
will die.1 Renal cell carcinoma occurs in a male-female ratio of
1.7:1, with peak incidence in the sixth to eighth decades of life.1-4
It is more prevalent in persons of northern European ancestry than in those
of Asian or African descent.2 There has been a steady increase in
the incidence of renal cell carcinoma over the past several decades, which may
be partly explained by greater use of diagnostic imaging. In addition,
mortality rates among patients with renal cell carcinoma have increased over
the past two decades.2,3
Etiology and Risk Factors
Renal cell
carcinoma commonly occurs in sporadic form, and only 1% to 4% of renal cell
carcinoma cases are associated with an inherited syndrome.4 While
the specific etiology of sporadic renal cell carcinoma is unknown, several
possible causal factors have been examined.2,5
Cigarette smoking contributes
to 24% to 30% of renal cell carcinoma cases. Additionally, persons with cystic
kidney disease undergoing chronic dialysis are estimated to have a 30- to
100-fold increased risk of renal cell carcinoma.2,5 Other risk
factors include hypertension, diuretic use, phenacetin use, and obesity.
Environmental and occupational exposure to toxins may also predispose persons
to an increased risk of renal cell carcinoma, although this has not been
proved with absolute certainty.5
Renal cell carcinoma is also
associated with von Hippel-Lindau (VHL) disease, an autosomal-dominant
disease. Evidence has shown that the VHL gene is mutated in a high percentage
of tumors among patients with sporadic clear cell renal carcinoma.2,5,6
Other chromosomal abnormalities, such as deletions and translocation of
chromosome 3p, are also associated with VHL disease and sporadic renal cell
carcinoma.2
Symptoms and Clinical
Presentation
A variety of
symptoms of renal cell carcinoma may occur, although most patients remain
asymptomatic until the disease becomes advanced. Hematuria (i.e., blood in the
urine) is the most common symptom upon clinical presentation. Pain, hematuria,
and an abdominal or flank mass occur in approximately 10% of patients. This
classic triad of symptoms is indicative of metastatic disease and is
associated with a poor prognosis. Other common symptoms include
normocytic/normochromic anemia, fever, and weight loss. Less common but often
described symptoms include hypercalcemia, polycythemia, and hepatic
dysfunction not associated with liver metastases.2,5
Forty-five percent of patients
present with localized disease, while 25% present with locally advanced
disease, and 30% present with metastatic disease. The most common sites for
metastases are lung, soft tissues, bone, and liver.5
Diagnosis
Currently, there
are no recommended screening modalities for the early diagnosis of renal cell
carcinoma. Diagnosing suspected renal cell carcinoma begins with a careful
medical history evaluation and a physical examination. The medical history
assessment should include the identification of potential risk factors as well
as an evaluation of the patient's symptoms.
If renal cell carcinoma is
suspected, additional studies should be performed. Radiographic evaluation can
determine the presence of a renal mass and aid in diagnosis. There is no
single imaging method that is best for diagnosing renal cell carcinoma.
Abdominal and pelvic CT, renal ultrasound, renal arteriography, renal
venography, and abdominal and pelvic MRI can each provide unique information
regarding tumor size and extent of extrarenal disease. Multiple imaging
techniques are often utilized to provide the greatest amount of information
possible.2,5
Contrast-enhanced CT is the
technique of choice for imaging a renal mass because it is able to provide
information about renal artery and vein involvement as well as local lymph
node involvement, and because it can usually differentiate cystic masses from
solid masses.2,5 CT can provide the following information about a
tumor: size, location, relationship to local vessels, presence of
extracapsular spread, and whether there is invasion to adjacent organs.
Ultrasound provides
information about extrarenal extension of tumor, lymph node involvement,
adrenal involvement, and infiltration of adjacent viscera. Ultrasound is also
useful for differentiating between solid and cystic masses.
MRI may be helpful when
ultrasound and CT are nondiagnostic and/or when radiographic contrast cannot
be administered due to poor renal function or allergy. MRI may also be helpful
in identifying the extent of involvement with the nephron collecting system or
inferior vena cava, showing local anatomy, and identifying whether direct
capsular spread is present.2
Renal arteriography is not
commonly used to diagnose renal cell carcinoma but may be useful in patients
with a small, indeterminate renal mass or for defining large tumor vasculature
in patients undergoing surgery.
An evaluation of
extra-abdominal disease sites must also be done to look for potential
metastatic tumor sites. Lung or bone involvement can be identified with the
use of chest x-ray. Chest CT is usually unnecessary if chest x-ray results are
normal. If a patient has an elevated alkaline phosphate level or symptoms
suggestive of bone metastases, a bone scan should be performed.2
Head CT might also be performed to evaluate whether brain metastases are
present.
Histology
Pathologic
classification of kidney cancers is based upon cell of origin and tumor
location.6 Eighty-five percent to 90% of kidney cancers are renal
cell carcinomas that begin within renal cortex epithelium.3,6 There
are a number of different histology types that occur in renal epithelium
malignancies; 75% of cases are of clear cell histology, 15% are of either type
1 or type 2 papillary histology, and 5% are of chromophobe and oncocytic
histology.5 Each histology has a different clinical course and is
caused by different genes. In general, clear cell carcinomas tend to behave
aggressively, while chromophobic carcinomas are more indolent.7
Staging and Prognosis
Historically, the
most common staging system used was the Robson modification of the Flocks and
Kadesky system.5 In the mid-1980s, the tumor, nodes, and metastases
(TNM) classification system was introduced and validated by the American Joint
Committee on Cancer.7 By the mid to late 1990s, the TNM system, a
more accurate method for classifying the extent of tumor involvement, took
precedence for kidney cancer staging.
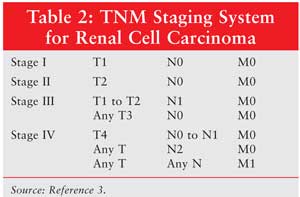
The TNM system classifies
cancer stages based on the size of the tumor, the extent of local invasion,
the number of regional positive lymph nodes, and the presence of distant
metastasis (Tables 1 and 2). TNM stage is the most consistent
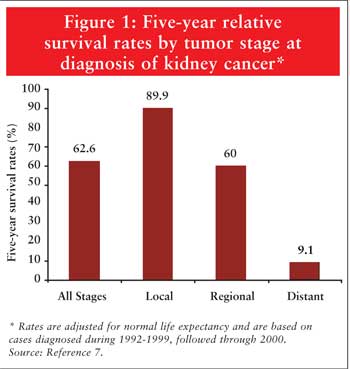
variable determining patient prognosis. Survival decreases as stage of disease
increases (Figure 1).5,7 The majority of patients are
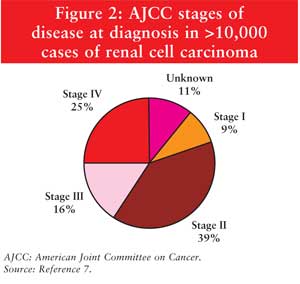
diagnosed with localized disease, as seen in Figure 2.
Another important variable in
prognosis is the performance status of the patient, which is commonly assessed
using the Eastern Cooperative Oncology Group (ECOG) performance status scale (
Table 3).7 Patients with an ECOG performance status score from
0 to 2 may be more likely to tolerate aggressive therapy, whereas patients
with a performance status score of 3 or 4 may not be able to tolerate surgery
or other therapies.5,7


Other factors that influence
survival include clinical signs and symptoms (fever and weight loss),
laboratory values (e.g., serum lactate dehydrogenase, serum calcium
concentration, and hemoglobin level), and tumor grade and histology.5,7,8
Progression-free interval is also an indicator of survival. The longer the
interval between definitive treatment and disease recurrence, the longer the
projected survival.3 Factors influencing prognosis are listed in
Table 4.
The University of California,
Los Angeles, Integrated Staging System (UISS) incorporates histological grade
and performance status into the TNM classification system.5,7
Although not widely used currently, integrated staging systems may be used
with more frequency in the future.

Treatment
Management options
for renal cell carcinoma are determined by stage of disease.3
Localized (Stage I-III)
Renal Cell Carcinoma
Surgery:
Surgery is the only known curative therapy for early-stage renal cell
carcinoma. Radical nephrectomy, which includes complete removal of the
Gerota's fascia, kidney, and ipsilateral adrenal gland, is considered the gold
standard.5,7,9
Most patients who undergo
surgery as treatment for localized disease are able to achieve disease
control. About 20% to 30% of patients experience relapse.3 Most
relapses occur systemically, with metastases in the lungs being the most
common distant site and occurring in more than half of patients.3
Less than 2% of radical nephrectomy patients and approximately 3% of partial
nephrectomy patients experience local recurrence (i.e., recurrence at the
operative site or regional lymph nodes).7 Most relapses occur
within three years of nephrectomy, with a median time to relapse of one to two
years. Local recurrences can be successfully treated with surgery. In patients
who may be left with little or no renal function following radical
nephrectomy, partial nephrectomy may help to preserve the kidney to ensure
adequate kidney function.10
Adjuvant Therapy:
There is no role for adjuvant therapy in patients with localized renal
carcinoma except under investigational protocol. Radiation, chemotherapy, and
biologic or immunomodulating therapies have not been shown to reduce relapse
rates following definitive surgical treatment. Observation after nephrectomy
is currently the standard of care for disease stages I, II, and III.3
Advanced (Stage IV) Renal
Cell Carcinoma
Surgery:
Surgery as palliative therapy may be considered for a select group of
patients. Patients with a solitary site of metastasis or patients with a
solitary recurrence following nephrectomy are considered surgical candidates.
Additionally, patients with symptoms related to the primary tumor and
bulkiness of disease--such as fever, nausea, pain, or gastrointestinal
obstruction--may have a nephrectomy to improve their quality of life.
3
Neoadjuvant Therapy:
Improvement in survival has been seen with the administration of
immunomodulating therapy before surgery.3,5 A pooled analysis from
two trials showed a 5.8-month increase in median survival with the addition of
interferon-alpha before cytoreductive nephrectomy.11
Adjuvant Therapy:
The National Comprehensive Cancer Network (NCCN) Kidney Cancer Clinical
Practice Guidelines advise that patients with good prognostic features, good
performance status, and metastases only to the lung are most likely to benefit
from systemic therapy after nephrectomy.3
Chemotherapy:
Renal cell carcinoma is minimally responsive to chemotherapy. A comprehensive
review of 83 trials involving more than 4,000 patients reported a chemotherapy
response rate of 6%.12
Historically, patients have
shown some response to therapy with the single agents floxuridine,
5-fluorouracil, and vinblastine. Floxuridine and 5-fluorouracil are
antimetabolites that work by inhibiting thymidylate synthase, a pivotal enzyme
that catalyzes the de novo production of thymidylate and thymidine nucleotides
that are necessary for DNA synthesis.5 Vinblastine binds to
tubulin, causing inhibition of the mitosis phase of the cell cycle.5
A comprehensive review reported overall response rates of 43% or lower in
patients receiving floxuridine, an overall response rate of 10% in patients
taking 5-fluorouracil, and overall response rates of 7% or lower in patients
receiving vinblastine.5
More recently, single-agent
therapy with capecitabine or gemcitabine has shown some benefit when used in
patients with metastatic disease. A phase II study evaluating the use of
capecitabine in 26 patients in whom first- or second-line therapy with
immunotherapy had failed found that 8.7% of patients exhibited a partial
response.13 Another phase II trial evaluating the use of
gemcitabine in 37 patients with metastatic or inoperable renal cell carcinoma
yielded a response rate of 8.1%.14
Multiple studies have been
done evaluating combination chemotherapy regimens for advanced-stage renal
cell carcinoma. Clinical trials evaluating the safety and efficacy of
combinations using 5-fluoropyrimidines and gemcitabine are currently under
way. Two recent trials using different combinations of capecitabine and
gemcitabine have reported a partial response rate of 15%, with 53% of patients
having stable disease.15,16 Another trial using a combination of
gemcitabine and 5-fluorouracil has demonstrated a partial response rate of 17%.
17 However, until more information is available, combination
chemotherapy for metastatic renal cell carcinoma remains investigational.
The chemoresistance exhibited
by renal cell carcinoma is unexplained, and consequently, unlike other solid
tumor treatments, chemotherapy is not commonly pursued as treatment for
advanced kidney cancer. The NCCN guidelines recommend single-agent
chemotherapy as an option for first-line treatment of metastatic renal cell
carcinoma in patients with non–clear cell histology.3
Hormone Therapy:
Since the 1960s, progestins and androgens have been evaluated for use in the
treatment of renal cell carcinoma. Medroxyprogesterone acetate has been used
in the past, but efficacy has been found to be poor, with an overall response
rate of 2%.5 This agent is not recommended as systemic therapy for
metastatic renal cell carcinoma.3
The antiestrogen tamoxifen has
also been studied as a treatment for metastatic renal cell carcinoma.
Unfortunately, response rates were low, ranging from 3% to 7%.5
Toremifene, another antiestrogen, showed an initial response rate of 17%;
however, additional studies have been unable to duplicate this response.5
Anti estrogens are not recommended as a treatment option for stage IV renal
cell carcinoma.3
Immunotherapy:
Renal cell carcinoma transiently evokes an immune response that results in
spontaneous and dramatic remissions. Unfortunately, review of cases of
spontaneous regression shows that the majority of them are short lived, with
duration of response lasting two to 13 months.5 Consequently, the
phenomenon of spontaneous regression should not be depended upon as therapy
for renal carcinoma.
The immune system is utilized
to treat renal cell carcinoma through multiple complex mechanisms.
Immunomodulating pharmacologic agents are used to stimulate the activity of
the immune system.
Interferon alpha was the first
cytokine to be investigated as a treatment for renal cell carcinoma.
Interferon alpha is a protein with the ability to enhance phagocytic activity
of macrophages, increase the cytotoxicity of natural killer cells, enhance the
major histocompatibility antigen presentation pathway, and inhibit cell
proliferation. Side effects of interferon alpha include fever, constitutional
symptoms, fatigue, injection-site reactions, myelosuppression, skin rash, and
neurologic effects. Constitutional symptoms and fatigue are considered to be
dose-limiting toxicities, and there is a black box warning associated with a
low incidence of life-threatening neuropsychiatric, autoimmune, ischemic, and
infectious disorders.5
A multivariate analysis in
which patients with advanced renal cell carcinoma were treated with interferon
alpha concluded that the overall response rate was approximately 10%, with a
median survival duration of 11.4 months and a five-year survival rate of 3%.
18 Additional studies have reported response rates of 29% or lower.5
Interferon alpha is not FDA
approved for renal cell carcinoma, but it is listed as a treatment option for
metastatic renal cell carcinoma in the NCCN guidelines.3 The
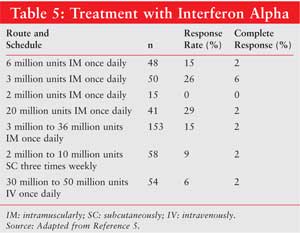
optimal dose, schedule, and route of administration for interferon alpha is
not known. Several regimens and their respective response rates are listed in
Table 5. Although a small but significant benefit has been seen with use
of interferon alpha, risks such as toxicity from chronic therapy and lack of
long-term benefit should be considered.
Another immunomodulating
therapy used for the treatment of renal cell carcinoma is interleukin-2
(IL-2). IL-2 is an autocrine factor with several immunoregulatory properties
that influences the activity of T cells and natural killer cells. Recombinant
human IL-2, known as Aldesleukin, is produced by genetically engineering
bacteria. Aldesleukin is one of the few agents approved by the FDA for the
treatment of metastatic renal cell carcinoma.
IL-2 may be administered in
varying doses and schedules.3,5 Several regimens and their
respective response rates are listed in Table 6. High-dose regimens of
IL-2 have demonstrated remission rates of 10% to 20%, with a median duration
of response of 19 to 91 months.11 Unfortunately, the high-dose
regimen is associated with several severe side effects, including severe
hypotension and tachycardia, capillary leak syndrome, infection, myocardial
infarction, pulmonary edema, renal insufficiency, hepatic insufficiency, and
central nervous system disturbances.5 Constitutional symptoms are
also common. Due to the severity of side effects, patients receiving high-dose
IL-2 are treated in an ICU setting, where frequent monitoring is available and
supportive care measures can be rapidly initiated.5
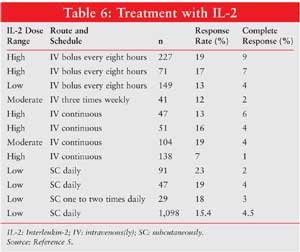
Because of the toxicity
associated with high-dose IL-2 regimens, low-dose regimens have been
investigated. Several studies have shown widely varied response rates, with
few durable or complete responses. Comparative studies between high- and
low-dose IL-2 regimens have shown that high-dose IL-2 is superior in terms of
response rate and durability of response. Low-dose IL-2 as single-agent
therapy is not recommended as first-line therapy.3,5
IL-2 has also been studied in
combination with interferon alpha with hopes of achieving a synergistic
effect, since both have demonstrated benefit when used alone. Higher response
rates are achieved with combination therapy (18.6%) when compared to either
agent alone (6.5% to 7.5%).3 However, combination therapy is
associated with greater toxicity and has failed to demonstrate an improvement
in survival. IL-2 and interferon-alpha combination therapy may be a reasonable
option as first-line therapy in metastatic renal cell carcinoma as recommended
by the NCCN guidelines.3
Targeted Therapy:
Recent advances in the understanding of the biology of renal cell carcinoma
have led to the development of new treatments for metastatic disease. Renal
cell carcinoma is a highly vascularized tumor with high vascular endothelial
growth factor (VEGF) and endothelial growth factor receptor (EGFR) expression.
5,10,13 Tumor growth is also mediated through multiple signaling
pathways. Intravenous and oral agents have been developed that specifically
act upon these molecular targets.
Sorafenib (Nexavar) is an
orally bioavailable agent that inhibits multiple kinases involved in tumor
proliferation and angiogenesis.19,20 Specifically, it inhibits VEGF
receptors 2 and 3, platelet-derived growth factor (PDGF) receptor beta, stem
cell factor receptor (KIT), and Fms-like tyrosine kinase 3 (FLT3).19,20
Sorafenib is FDA approved for the treatment of metastatic renal cell
carcinoma.20
A phase II trial and a phase
III trial have evaluated the use of sorafenib in metastatic renal cell
carcinoma.21,22 Both studies showed that sorafenib significantly
improved progression-free survival compared with placebo (163 to 167 days vs.
41 to 84 days).21,22 Neither trial showed statistically significant
improvement in overall survival. Common side effects included hypertension,
fatigue, rash, hand-foot syndrome, alopecia, diarrhea, and nausea. The
approved dose of sorafenib is 400 mg orally twice daily.20
Sunitinib (Sutent) is another
orally bioavailable agent that inhibits multiple kinases involved in tumor
growth and angiogenesis.19,23 It inhibits VEGF receptors 1, 2, and
3, PDGF receptors alpha and beta, KIT, and FLT3. Sunitinib is FDA approved for
the treatment of metastatic renal cell carcinoma and gastrointestinal stromal
tumors.23
Two phase II clinical trials
have evaluated response rates and durability of response to sunitinib.24
Twenty-five percent to 36% of patients had an objective response lasting from
27 to 54 months. There is no information regarding overall survival benefit
with use of sunitinib. Common side effects include diarrhea, nausea, fatigue,
rash, skin discoloration, and hypertension.19,24 The approved dose
of sunitinib is 50 mg orally once daily for four weeks followed by two weeks
off.24
Bevacizumab (Avastin) is a
recombinant monoclonal antibody that binds to VEGF, preventing it from binding
to the VEGF receptor and inhibiting angiogenesis.16 It is under
investigation for use in metastatic renal cell carcinoma.
Two phase II trials have shown
benefit of bevacizumab, either as a single agent or in combination with
erlotinib.25,26 Single-agent bevacizumab improved median time to
tumor progression from 2.5 to 4.8 months when compared to placebo.25
The effective dose of bevacizumab used in this trial was 10 mg/kg given
intravenously every 14 days. Common side effects of bevacizumab in this
setting include fatigue, hypertension, proteinuria, and epistaxis. Patients
receiving bevacizumab may also have an increased risk of hemorrhage,
thrombosis, gastrointestinal perforation, and myocardial infarction.
The NCCN guidelines recommend
sorafenib and sunitinib as first-line agents for treatment of metastatic renal
cell carcinoma. Bevacizumab is not FDA approved for renal cell carcinoma, but
it is recommended by the NCCN guidelines as single-agent, second-line therapy,
only after first-line therapy with another agent has failed.3
Clinical Trial
Enrollment:
Because
of minimal responsiveness to conventional cytokine therapy and the poor
survival associated with metastatic disease, it is a high priority to identify
more effective agents through clinical investigation. Enrollment in clinical
trials is the preferred option for patients who relapse following resection or
who have metastatic disease.3
Conclusion
Renal cell
carcinoma is a relatively rare malignancy. Surgery is the only known curative
modality for patients with stage I, II, or III disease. Despite several
treatment advancements in the past few years, patients with advanced stages of
renal cell carcinoma will eventually die from their illness. Currently, IL-2,
sorafenib, and sunitinib are the only agents that are FDA approved for the
treatment of advanced renal cell carcinoma. Several other agents, including
interferon alpha and several chemotherapy drugs, are recommended by the NCCN
guidelines for use in stage IV disease. Additional clinical trials with
investigational agents and combination regimens with chemotherapy are needed
to improve treatment of renal cell carcinoma.
References
1. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106-130.
2. Redman BG, Kawachi M, Hurwitz M. Urothelial and kidney cancers. In: Pazdur R, Coia LR, Hoskins WJ, eds. Cancer Management: A Multidisciplinary Approach. 9th ed. Sudbury, MA: Jones and Bartlett Publishers; 2005:429-444.
3. Clinical Practice Guidelines in Oncology: Kidney Cancer, version 2.2006. National Comprehensive Cancer Network Web site. Available at: www.nccn.org/professionals/physician_gls/PDF/kidney.pdf. Accessed September 15, 2006.
4. Pavlovich CP, Schmidt LS. Searching for the hereditary causes of renal-cell carcinoma. Nat Rev Cancer . 2004;4:381-393.
5. Linehan WM, Bates SE, Yang JC. Cancer of the kidney. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:1140-1164.
6. Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477-2490.
7. Drucker BJ. Renal cell carcinoma: current status and future prospects. Cancer Treat Rev. 2005;31:536-545.
8. Lam JS, Shvarts O, Leppert JT, et al. Renal cell carcinoma 2005: new frontiers in staging, prognostication and targeted molecular therapy. J Urol. 2005;173:1853-1862.
9. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655.
10. Vogelzang NJ, Stadler WM. Kidney cancer. Lancet. 1998;352:1691-1696.
11. Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071-1076.
12. Amato RJ. Chemotherapy for renal cell carcinoma. Semin Oncol. 2000;27:177-186.
13. Wenzel C, Locker GJ, Schmidinger M, et al. Capecitabine in the treatment of metastatic renal cell carcinoma failing immunotherapy. Am J Kidney Dis. 2002;39:48-54.
14. Rohde D, De Mulder PH, Weissbach L, et al. Experimental and clinical efficacy of 2'-2'-difluorodeoxcytidine (gemcitabine) against renal cell carcinoma. Oncology. 1996;53:476-481.
15. Waters JS, Moss C, Pyle L, et al. Phase II clinical trial of capecitabine and gemcitabine chemotherapy in patients with metastatic renal carcinoma. Br J Cancer. 2004;91:1763-1768.
16. Stadler WM, Halabi S, Rini B, et al. A phase II study of gemcitabine and capecitabine in metastatic renal cancer: a report of Cancer and Leukemia Group B protocol 90008. Cancer. 2006;107:1273-1279.
17. Rini BI, Vogelzang NJ, Dumas MC, et al. Phase II trial of weekly intravenous gemcitabine with continuous infusion fluorouracil in patients with metastatic renal cell cancer. J Clin Oncol. 2000;18:2419-2426.
18. Minasian LM, Motzer RJ, Gluck L, et al. Interferon alfa-2a in advanced renal cell carcinoma: treatment results and survival in 159 patients with long-term follow-up. J Clin Oncol. 1993;11:1368-1375.
19. Patel PH, Chaganti RS, Motzer RJ. Targeted therapy for metastatic renal cell carcinoma. Br J Cancer. 2006;94:614-619.
20. Nexavar (sorafenib tablets) [package insert]. West Haven, CT: Bayer; 2005.
21. Escudier B, Szczylik C, Eisen T, et al. Randomized phase III trial of the Raf kinase and VEGFR inhibitor sorafenib (BAY 43-9006) in patients with advanced renal cell carcinoma (RCC). J Clin Oncol. 2005;23(June 1 Supplement):LBA4510.
22. Ratain MJ, Eisen T, et al. Final findings from a phase II, placebo-controlled, randomized discontinuation trial (RDT) of sorafenib (BAY 43-9006) in patients with advanced renal cell carcinoma (RCC). J Clin Oncol. 2005;23(June 1 Supplement):4544.
23. Sutent (sunitinib malate capsules) [package insert]. New York, NY: Pfizer Inc.; 2006.
24. Motzer RJ, Rini BI, et al. Phase 2 trials of SU11248 show antitumor activity in second-line therapy for patients with metastatic renal cell carcinoma (RCC). J Clin Oncol. 2005;23(June 1 Supplement):4508.
25. Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427-434.
26. Hainsworth JD, Sosman JA, Spigel
DR, et al. Treatment of metastatic renal cell carcinoma with a combination of
bevacizumab and erlotinib. J Clin Oncol. 2005;23:7889-7896.
To comment on this article, contact
editor@uspharmacist.com.





