US Pharm. 2016;41(6):30-34.
ABSTRACT: Systemic lupus erythematosus (SLE) is an autoimmune disease that causes inflammation throughout the body. SLE frequently impacts many organ systems, including the integumentary, musculoskeletal, nervous, and cardiovascular systems. Because of the widespread impact of SLE on the body, a variety of medications are used to target the symptoms of the disease. Medications also have been developed that target the disease process. Although the prognosis of SLE has improved with the advent of better detection methods and enhanced treatment strategies, the need remains for a better understanding of the disease and more targeted treatment options.
Systemic lupus erythematosus (SLE) is an autoimmune disease that impacts many body systems, with a wide variety of signs and symptoms in each patient.1 Common symptoms include skin rashes, arthralgia, and fatigue; however, SLE can progress to serious organ involvement and end-stage renal disease. Patients with SLE experience periods of minimal or no disease activity (remission) and periods of increased activity (flares).2 Treatment of SLE helps patients remain in a state of remission by preventing flares, and any flares that do occur are addressed quickly. Treatment options depend on the organ systems involved and the severity of symptoms.
Epidemiology
SLE most commonly occurs in women of childbearing age.2 The female-to-male ratio is estimated to be 9 to 1.3 It is estimated that >300,000 individuals in the United States have SLE.1 The prevalence is estimated to be about 52.2 per 100,000 persons. The incidence is higher in persons of African-American and African-Caribbean descent.3
SLE is believed to develop as a result of genetic and environmental influences. Many genes have been linked to SLE, and it appears that different genes may impact how the disease presents in individual patients. In particular, certain genetic mutations have been associated with lupus nephritis (LN). Specific genetic factors have been evaluated in terms of their relation to other disease manifestations, such as neuropsychiatric symptoms, cutaneous symptoms, hematologic symptoms, and others.4 Some of the best-studied genetic links are integrin-alphaM, Fc-gamma receptor, PRDM1-ATG5, HLA-DR2, HLA-DR3, and TNFAIP3.3,4
The best-studied environmental trigger is ultraviolet light, which worsens the cutaneous manifestations of the disease. Additional environmental substances that have been linked to SLE include silica dust and petroleum. Hormones also appear to have an influence on disease, with estrogen a possible cause of increases in flares. Other potential causes include vitamin D deficiency, infections, and pesticide exposure.3
Clinical Features
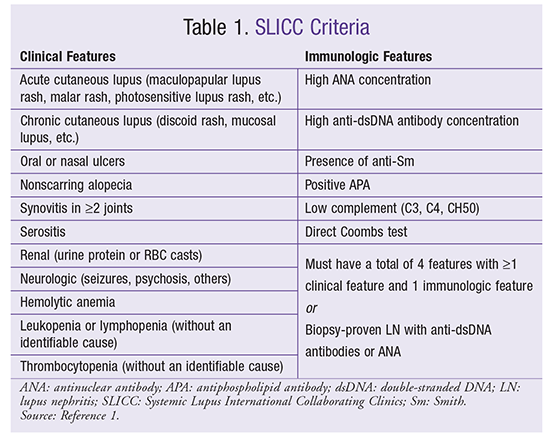
SLE is a disease of many features. In the past, criteria developed by the American College of Rheumatology in 1982 and revised in 1997 were used to classify SLE. Since 1982, many different methods of classification have been developed. The most recent criteria were published by the Systemic Lupus International Collaborating Clinics (SLICC) in 2012. The SLICC criteria use a combination of clinical and immunologic features to determine whether a patient has SLE. Patients must have a total of four criteria, with at least one clinical feature and one immunologic feature. However, a patient with LN proven via biopsy as well as antinuclear antibody (ANA) or anti-dsDNA (double-stranded DNA) antibody testing would automatically be identified as having SLE regardless of any other clinical or immunologic criteria.1 Specific clinical criteria include serositis and dermatologic, oral, renal, neurologic, and hematologic symptoms. Immunologic characteristics include high ANA and anti-dsDNA, presence of anti-Smith (anti-Sm) proteins, antiphospholipid antibodies (APAs), low complement, and direct Coombs test. It is not necessary that all four criteria be present at once; if the patient has a history of these criteria at varying times, that is sufficient for the classification.1 TABLE 1 delineates the SLICC criteria.
In addition to the features noted in the SLICC criteria, patients with SLE frequently experience fatigue. The prevalence has been reported to be 50% to 90%, with >50% of patients stating that fatigue is the most debilitating feature of the disease.5
Because each SLE patient presents differently, the prognosis will depend on the individual and the severity of his or her symptoms. In general, patients with extensive organ involvement have the worst prognosis.2 Patients with early disease onset also have poorer outcomes. The mortality risk continues to decline as advances in therapy continue. A trial from 2009 suggested that the 10-year survival rate is 92%.3
Types of SLE
Some patients have specific disease manifestations that identify them as having certain types of SLE. These types include neuropsychiatric lupus, cutaneous lupus erythematosus, and LN. Each type of lupus has its own treatment recommendations.2 LN is associated with higher rates of morbidity and mortality.4 Owing to the frequent presence of APAs, antiphospholipid syndrome is a concern in patients with SLE.2
Nonpharmacologic Therapy
There are many nonpharmacologic recommendations that can help patients better manage SLE.
Sunscreen: Many of the cutaneous manifestations of SLE are triggered by sunlight. It is important to recommend that the patient wear sunscreen daily.2
Diet: Owing to the comorbid cardiovascular (CV) disease frequently seen in patients with SLE, diet is an active area of research. One trial examined a low-glycemic-index diet versus a low-calorie diet in SLE patients and found that both groups experienced weight loss, had no increase in disease activity, and demonstrated a significant decline in fatigue.6 The trial, which lasted 6 weeks, did not show a significant change in CV or glycemic outcomes. Results of a study evaluating several B vitamins and fiber suggested an inverse relationship between intake of vitamin B6 and fiber and SLE activity.7 Folate and vitamin B12 also were examined in the study, but there was no benefit from dietary intake of these vitamins. The same authors also investigated vitamin C in a separate study and found that dietary intake had an inverse relationship with disease activity.8
Psychological Support: Depression is a common comorbidity in SLE, and patients should be screened regularly.3 Additionally, fatigue often impacts daily functioning. Support groups, counseling, and biofeedback are techniques that may be helpful for patients with SLE.3,5
Exercise: Regular exercise helps patients with SLE achieve a better quality of life, including improving symptoms of depression.3 Routine exercise also benefits the common comorbidity of CV disease and helps combat SLE-associated fatigue.5
Acupuncture: Small clinical trials evaluating the efficacy of acupuncture in patients with SLE have demonstrated improvements in fatigue and pain; however, a decrease in disease activity has not been proven.5,8 Overall, clinical trials suggest a possible benefit in patients experiencing musculoskeletal pain; however, larger trials are needed to prove the advantage for SLE.8
Pharmacotherapy
Treatment options for SLE depend on the patient’s symptoms and disease severity. Common treatment options include antimalarial agents, glucocorticoids, nonsteroidal anti-inflammatory drugs (NSAIDs), and immunosuppressive medications.2
Hydroxychloroquine: This agent is used for mild disease activity and disease maintenance. It is initially dosed at 400 mg once or twice daily. After several weeks to months, depending on patient response, the lowest effective dosage is used (200 to 400 mg once or twice daily; some patients may be able to take even a lower dosage).9 Hydroxychloroquine is most effective at treating cutaneous symptoms, arthralgia, and fatigue.
Prednisone: This corticosteroid is used for moderate-to-severe disease activity. The dosage depends on the severity of the activity, but usually ranges from 5 to 50 mg daily. Prednisone addresses inflammation throughout the body.3
NSAIDs: The NSAID class is administered for its antipyretic and anti-inflammatory properties. For this reason, NSAIDs are useful for treating symptoms of inflammation and fever.3
Belimumab: Belimumab is a monoclonal antibody (Mab) that inactivates B-cell activating factor.3 Belimumab, which is administered IV, is dosed initially at 10 mg/kg every 2 weeks for three doses. After the first three doses, the medication is administered at the same dosage every 4 weeks.9 The most common adverse reactions include arthralgia, upper respiratory tract infections, headache, fatigue, and nausea.10 Possible serious adverse reactions include infusion reactions and psychiatric symptoms including suicidal ideation.5,11 Belimumab reduces the need for corticosteroids and is effective against a variety of symptoms, such as musculoskeletal and serologic concerns.11 A recent meta-analysis confirmed the efficacy of belimumab in combination with standard therapy versus standard therapy with placebo.10
Azathioprine: This agent may be used for moderate-to-severe disease, with the dose based on disease severity.3 The dosage typically ranges from 1.5 to 2 mg/kg daily.9
Methotrexate (MTX): MTX is used for moderate-to-severe disease, with the dose dependent on the severity of disease activity.3 MTX has a starting dosage of 7.5 mg once weekly and is increased by 2.5 mg each week. The recommended maximum is 20 mg once weekly.9 In particular, MTX is used for cutaneous and joint symptoms.3
Mycophenolate mofetil: This medication is used for moderate-to-severe disease. The dosage ranges from 750 mg to 3 g, depending on disease severity.3
Cyclophosphamide: Cyclophosphamide is used for severe disease activity and LN.3 The dosage for LN is 500 mg every 2 weeks for six doses or 500 to 1,000 mg/m2 once every 6 months for six doses. Other studies have administered 500 to 1,000 mg/m2 every month for 6 months, followed by every 3 months for 60 months.9
Cyclosporine: This agent is used for moderate-to-severe disease.3 For LN, cyclosporine is initiated at 4 mg/kg daily. After 1 month, the dose is titrated down by 0.5 mg/kg every 2 weeks to a maintenance dosage of 2.5 to 3 mg/kg daily.9
Tacrolimus: This calcineurin inhibitor is used for LN and cutaneous symptoms.3 Tacrolimus has been used in clinical trials at a dosage of 0.05 to 0.1 mg/kg daily.12,13 Topically, tacrolimus is used as a 0.1% ointment twice daily.14
Leflunomide: Leflunomide is a disease-modifying agent that helps suppress the immune system.3,9 A clinical trial studying the efficacy of leflunomide used a loading dose of 1 mg/kg daily for 3 days followed by 30 mg daily.15
Drugs in Research
Many drugs for the treatment of SLE are in various stages of development. This includes novel medications, as well as agents that are currently approved for other indications. Some of the drugs in development are discussed next.3
B-cell Activity: Medications that are being studied to target B-cell activity include rituximab, epratuzumab, atacicept, and bortezomib. Currently, one medication that targets B-cell activity—belimumab—is approved. Rituximab, an Mab, causes B-cell depletion and has undergone numerous clinical trials evaluating its efficacy in SLE. Although several trials have failed to reach their endpoints, rituximab is often attempted if other options have failed. Rituximab may be more efficacious in patients with LN.3 A clinical trial of atacicept has suggested a benefit; however, the arm of the trial that demonstrated a potential advantage was discontinued early owing to an increased risk of infections.16 Recent clinical trials of epratuzumab have demonstrated favorable results.17
T-cell Activity: Potential SLE treatment options that inhibit T-cell function include abatacept, ruplizumab, toralizumab, and rigerimod.3 Clinical trials investigating the use of abatacept have failed to meet their endpoints; however, they have demonstrated a trend indicating that the drug may have some benefit, and more clinical trials are warranted.3,11
Interleukin-6: Tocilizumab is an Mab that targets interleukin-6. A pilot study of tocilizumab suggested clinical and serologic benefits; however, the adverse reaction of dose-related neutropenia may limit the utility of this medication in SLE.11
Tumor Necrosis Factor-alpha Inhibitors: These agents are approved for other disease states, but they are being studied for their potential benefits in SLE. Two that currently are under investigation are infliximab and etanercept.3
Type 1 Interferon (INF)-alpha Inhibitors: Two medications that work as type 1 INF-alpha inhibitors are sifalimumab and rontalizumab. A recent phase IIb trial suggests a potential benefit of sifalimumab for SLE, although there is an increased risk of infection.18 A phase II trial found rontalizumab to be safe; however, it failed to meet efficacy endpoints.19
Complement Inhibitors: Eculizumab is a drug that inhibits complement.3 Several studies of eculizumab have suggested a possible benefit for thrombotic microangiopathy, antiphospholipid syndrome, and LN; however, no clinical trials are under way.20-22
Dietary Supplements
Complementary and alternative medicine (CAM) is used in about 50% of patients with SLE. Most patients who use CAM to treat SLE are using dietary supplements.8
Vitamin D: Patients with SLE are at increased risk for vitamin D deficiency because of their SLE medications, sun avoidance and sunscreen use, and renal insufficiency.5 Evidence suggests that vitamin D supplementation may reduce disease activity.8 Vitamin D supplementation is well tolerated in patients with SLE.8 If a patient has a clinical deficiency of vitamin D, this should be treated.
Didehydroepiandrosterone (DHEA): Patients with SLE have been shown to have low levels of DHEA. DHEA supplementation may help decrease disease activity and improve quality of life; however, patients must be cautioned about the many side effects of DHEA use, such as acne, hirsutism, and weight gain.5,8
Omega-3 Fatty Acids: Patients with SLE may find omega-3 fatty acids beneficial because of their CV benefits and anti-inflammatory properties. Clinical trials have demonstrated mixed results; however, patients seem to tolerate the supplements well.8
Turmeric (Curcumin): This dietary supplement is known to have anti-inflammatory properties, which makes it potentially beneficial for diseases involving inflammation. One small trial saw some benefits of turmeric supplementation; however, larger trials are needed to confirm these results. No adverse events were noted in the trial.8
Immunizations
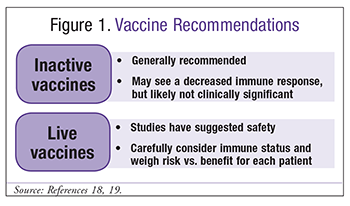
Patients with SLE are at increased risk for infections; therefore, it is important to consider the efficacy and safety of immunizations in these patients.22 The European League Against Rheumatism developed 13 recommendations, including general recommendations and some recommendations specific to certain vaccines, for patients with autoimmune inflammatory diseases such as SLE. In general, inactivated vaccines are considered safe in SLE patients; however, they may not elicit as robust an immune response as they would in a patient without SLE.23 Patients receiving a therapy that impacts B cells may have an especially low immune response. Therefore, it is recommended that patients be immunized either prior to initiating a therapy that impacts B cells or after the therapy is completed. Live vaccines require special consideration of the benefits and risks of immunization.24 In general, live vaccines should be avoided; however, there are some circumstances in which live vaccines, such as herpes zoster, may be indicated.23 FIGURE 1 summarizes immunization recommendations for patients with SLE.
Pregnancy and Breastfeeding
Patients with SLE who are pregnant or wish to become pregnant require special consideration. A study of 385 pregnant patients with SLE found that 81% of those with mild-to-moderate disease had good pregnancy outcomes.25 The rate of flares was 2.5% during the second trimester and 3% in the third trimester. Patients taking antihypertensive agents, those with low complement, and those with low platelet count were at highest risk for adverse pregnancy outcomes.25 Agents that have been used in pregnancy include hydroxychloroquine, low-dose aspirin, nonfluorinated glucocorticoids, azathioprine, and cyclosporine A. However, clear safety data are not available. Medications that should be avoided during pregnancy include mycophenolate mofetil, cyclophosphamide, and MTX.2
Hydroxychloroquine is considered an acceptable choice for patients who are breastfeeding, and low-dose prednisone and ibuprofen are often used. Additionally, azathioprine and MTX may be safe during breastfeeding. There are no current data on the safety of belimumab during breastfeeding, but it is expected that transmission through breast milk is minimal. Cyclophosphamide and mycophenolate mofetil should be avoided during breastfeeding.26
Conclusion
The treatment of SLE has improved with the additional use of immunosuppressive medications; however, there is still a need for targeted therapies. As research achieves a better understanding of the pathogenesis of SLE, the search for targeted therapies will become more precise. At this time, many clinical trials are under way that may change the future of how SLE is treated and improve disease outcome. In addition to the treatment of SLE, it is important to remember to counsel the patient about lifestyle modification and methods of preventing disease through immunization.
REFERENCES
1. Petri M, Orbai A, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686.
2. Bertsias G, Ioannidis JP, Boletis J, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67:195-205.
3. Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384:1878-1888.
4. Ceccarelli F, Perricone C, Borgiani P, et al. Genetic factors in systemic lupus erythematosus: contribution to disease phenotype. J Immunol Res. 2015;2015:745647.
5. Yuen HK, Cunningham MA. Optimal management of fatigue in patients with systemic lupus erythematosus: a systematic review. Ther Clin Risk Manag. 2014;10:775-786.
6. Davies RJ, Lomer MCE, Yeo SI, et al. Weight loss and improvements in fatigue in systemic lupus erythematosus: a controlled trial of a low glycaemic index diet versus a calorie restricted diet in patients treated with corticosteroids. Lupus. 2012;21:649-655.
7. Minami Y, Hirabayashi Y, Nagata C, et al. Intakes of vitamin B6 and dietary fiber and clinical course of systemic lupus erythematosus: a prospective study of Japanese female patients. J Epidemiol. 2011;21:246-254.
8. Greco CM, Nakajima C, Manzi S. Updated review of complementary and alternative medicine for systemic lupus erythematosus. Curr Rheumatol Rep. 2013;15:378.
9. Lexi-Drugs Online. Hudson, OH: Lexi-Comp, Inc; 2016. online.lexi.com. Accessed May 19, 2016.
10. Wei LQ, Liang YG, Zhao Y, et al. Efficacy and safety of belimumab plus standard therapy in patients with systemic lupus erythematosus: a meta-analysis. Clin Ther. March 23, 2016 [Epub ahead of print].
11. Grech P, Khamashta M. Targeted therapies in systemic lupus erythematosus. Lupus. 2013;22:978-986.
12. Chen W, Tang X, Liu Q, et al. Short-term outcomes of induction therapy with tacrolimus versus cyclophosphamide for active lupus nephritis: a multicenter randomized clinical trial. Am J Kidney Dis. 2011;57:235-244.
13. Moc CC, Ying KY, Yim CW, et al. Tacrolimus versus mycophenolate mofetil for induction therapy of lupus nephritis: a randomised controlled trial and long-term follow-up. Ann Rheum Dis. 2016;75:30-36.
14. Kuhn A, Gensch K, Haust M, et al. Efficacy of tacrolimus 0.1% ointment in cutaneous lupus erythematosus: a multicenter, randomized, double-blind, vehicle-controlled trial. J Am Acad Dermatol. 2011;65:54-64.
15. Wang HY, Cui TG, Hou FF, et al. Induction treatment of proliferative lupus nephritis with leflunomide combined with prednisone: a prospective multi-centre observational study. Lupus. 2008;17:638-644.
16. Isenberg D, Gordon C, Licu D, et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann Rheum Dis. 2015;74:2006-2015.
17. Strand V, Petri M, Kalunian K, et al. Epratuzumab for patients with moderate to severe flaring SLE: health-related quality of life outcomes and corticosteroid use in the randomized controlled ALLEVIATE trials and extension study SL0006. Rheumatology (Oxford). 2014;53:502-511.
18. Khamashta M, Merrill JT, Werth VP, et al. Sifalimumab, an anti-interferon-a monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis. March 23, 2016 [Epub ahead of print].
19. Kalunian KC, Merrill JT, Maciuca R, et al. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-a) in patients with systemic lupus erythematosus (ROSE). Ann Rheum Dis. 2016;75:196-202.
20. Pickering MC, Ismajli M, Condon MB, et al. Eculizumab as rescue therapy in severe resistant lupus nephritis. Rheumatology (Oxford). 2015;54:2286-2288.
21. El-Husseini A, Hannan S, Awad A, et al. Thrombotic microangiopathy in systemic lupus erythematosus: efficacy of eculizumab. Am J Kidney Dis. 2015;65:127-130.
22. Strakhan M, Hurtado-Sbordoni M, Galeas N, et al. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizumab: a case report and review of literature. Case Rep Hematol. 2014;2014:704371.
23. van Assen S, Agmon-Levin N, Elkayam O, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70:414-422.
24. Grein IH, Groot N, Lacerda MI, et al. HPV infection and vaccination in systemic lupus erythematosus patients: what we should really know. Pediatr Rheumatol Online. 2016;14:12.
25. Buyon JP, Kim MY, Guerra MM, et al. Predictors of pregnancy in patients with lupus: a cohort study. Ann Intern Med. 2015;163:153-163.
26. Noviani M, Wasserman S, Clowse ME. Breastfeeding in mothers with systemic lupus erythematosus. Lupus. February 16, 2016 [Epub ahead of print].
To comment on this article, contact rdavidson@uspharmacist.com.






