US Pharm. 2017;42(6)(Generic Drugs suppl):19-24.
In terms of social media posts, politics dominated the top 10 topics discussed in 2016. U.S. Pharmacist’s update on generic drugs always includes content that reflects political influence on these drugs, and the recent transition from one presidential administration to the next is a timely reminder of the potent impact politics has on healthcare policy. Just over 30 years ago, then-President Ronald Reagan signed the Hatch-Waxman Act (HWA), which was drafted with the intention of helping many Americans access generic medications and affordable, safe care. The HWA and many other acts—including the Affordable Care Act ushered in by then-President Barack Obama—are a reminder of how presidential administrations and enacted laws can change the climate of healthcare. Since 1984, more than 10,000 generic drugs have entered the market, and these products now represent roughly 50% of all prescriptions filled in the United States. Generic medicines constitute nearly 86% of the drugs dispensed in 2013, up from only 18% before the HWA.1
The name change of the Generic Pharmaceutical Association (GPhA) to the Association for Accessible Medicines (AAM) in February 2017 reflects a new identity that better represents the organization’s mission of making medications more accessible to individuals who need them. The AAM, a trade association that was founded in 2000, represents manufacturers and distributors of generic prescription drugs, manufacturers and distributors of bulk active pharmaceutical chemicals, and suppliers of other goods and services to the generic industry. According to its president and chief executive officer (CEO) Chester “Chip” Davis, the AAM also “improves the recognition that the generic and biosimilar medicines industry is one of the nation’s great health-care success stories.”2 Mr. Davis, who joined the GPhA/AAM in July 2015,3 is responsible for ensuring that the AAM serves “to improve the lives of patients and consumers by providing timely access to safe, effective, and affordable medicines.”4

The AAM actively engages in scientific, regulatory, health, and public-policy issues, so it was no surprise that it was keeping an eye on President Donald Trump’s healthcare agenda even before his inauguration in January 2017. Crucial to the evolution of public policy is the person appointed to lead a department or agency. For healthcare policy, that person is the secretary of the U.S. Department of Health and Human Services (HHS), Tom Price, who was named by President Trump and confirmed by the Senate in February 2017. The role of the HHS is to protect the health of—and provide essential human services to—all Americans, with particular focus on those who are most vulnerable and in greatest need of assistance. HHS programs impact outcomes of both public-health services and human services, including—but not limited to—promotion of patient safety and healthcare quality by ensuring the safety, effectiveness, quality, and security of foods, drugs, vaccines, and medical devices.5 When asked about the potential repeal of Obamacare, the AAM responded that the organization did not currently have a position on this.
Reaction and Comments by Chip Davis, AAM President and CEO
[In-person interview conducted on February 22, 2017] Q: In January 2017, just prior to the inauguration of President Donald Trump, you told the press that you were in favor of the nomination of Congressman Tom Price for HHS Secretary because of his background and expertise in complicated healthcare issues and because he continues to be a leader in providing affordable, high-quality healthcare in the U.S. Can you comment on your hopes for his future role and for the role of Congress, the Senate, and President Trump in the generic-drug industry?
A: President Trump has made clear that he intends to fight to reduce drug prices for patients across the U.S. The fact is that generics are 80% to 85% less expensive than brand drugs. And, while brand and specialty-medication prices continue to rise, the generic market experiences price deflation year after year. Express Scripts recently noted that brand-drug prices increased an average of 208% since 2008, while generics declined 74%.
President Trump understands from his business experience that increasing competition can drive down costs, and the Trump Administration has an opportunity to make more generic medicines available to Americans. Policymakers can take steps today to increase competition. Clearing the backlog of applications pending at the FDA and stopping certain brand-drug companies from blocking generic development would be a good start. Increasing generic competition should be part of any conversation about lowering drug costs for millions of Americans.
Q: In a recent press release, you mentioned that you look forward to advocacy by President Trump and the new administration to save costs, and you identify an area where much improvement is needed to reduce the backlog of generic-drug applications at the FDA. What is the current backlog of medications by number? Is this backlog getting worse, or has it been stable but suboptimal? Are any creative strategies being employed to improve generic utilization?
A: There is a backlog of more than 4,000 pending generic-drug applications at the FDA. Some progress is being made, but more can be done. The generic-drug industry will continue working collaboratively with the FDA on our shared goals, with the objective of ensuring more timely access to safe and effective generics and biosimilars.
Q: Your position to preserve and strengthen Medicare and Medicaid includes the offer to make policy suggestions on the use of generic drugs to continue to provide massive savings for each of these programs. Can you share some of the policy suggestions so that readers of this interview know what to advocate for beyond just the increased use of generics?
A: The AAM supports five policy prescriptions policymakers can take today that would boost generic and biosimilar competition:
1. Promote timely access to high-quality, affordable generic drugs that meet the standards of a streamlined development-and-approval process.
2. Create policies that recognize the different economic dynamics of the brand and generic markets.
3. Ensure an intellectual-property framework that balances the need for innovation with robust generic competition.
4. Stop some brand-drug companies from blocking or delaying generic-drug development.
5. Increase the use of affordable generics across all patient populations.
Q: AARP (formerly the American Association of Retired Persons) has been an ongoing resource for the AAM. AARP was founded in 1958 as the National Retired Teachers Association in order to promote the philosophy of productive aging and to address the need for health insurance for retired teachers. At that time, not only were older teachers without insurance, but nearly all older Americans were without it until 1965, when Medicare was enacted. In addition to working to enhance its partnerships within the aging community, AARP continues to focus on the founding principles of promoting independence, dignity, purpose, and quality of life in older adults. You recently quoted an AARP study on the escalation of generic-drug prices (up by 15% in the past year alone). Do you agree with this study?
A: Nearly every expert and supporting organization (such as AARP) agrees that generic drugs help lower drug costs. According to the HHS Assistant Secretary for Planning and Evaluation, a review of the evidence strongly supports the conclusion that generic-drug prices are not an important part of the drug-cost problem facing the nation. In fact, about two-thirds of generic products appear to have experienced price declines in 2014.6
Q: The AARP Brand Drug Pricing Report that the AAM has referenced in recent press releases reinforces the need for improved generic-drug utilization through increased competition. In addition to the observed increases in brand pharmaceutical prices (especially specialty drugs) for the past year, other AARP studies find that generic prices for many commonly used drugs are dropping, some by as much as 4%. The AAM notes that the AARP report found that the average senior citizen taking three prescriptions filled with an alternative generic product saved almost $850 annually. According to you, the AARP report confirms that the entire healthcare system (including seniors) would greatly benefit from more generic competition and that it urges the new administration and policymakers to take immediate action to stimulate competition. What specific actions can be taken to improve generic competition?
A: As stated earlier, on its RX Solutions website, the AAM offers five solutions that can be further discussed as paths to succeed in this generic initiative7:
1. Approve a Regulatory Pathway to Remove Duplicative International Drug-Approval Processes: There are European agencies that function to ensure drug safety and quality standards, just as the FDA does. These activities are often duplicative, costly, and unnecessary, so the AAM has suggested that there be a coordinated joint strategy to allow generic-drug manufacturers to follow a single pathway to provide generic-drug access to all consumers.
2. Repeal the Generic-Drug Penalty in Medicaid: Legislation has been introduced that would require generic-drug makers to provide larger Medicaid drug rebates whenever their product price rises faster than the rate of inflation. This “inflation penalty” has always applied to brand-name drugs only, so this bill would extend the requirement to manufacturers of generic drugs. One of the main concerns the AAM has expressed is that generic manufacturers’ supply and material costs fluctuate more than those of most brand-name companies and that they are also more fiscally sensitive to the percentage charged for the penalty.
3. Maintain Inter Partes Review (IPR) for Patents and Put an End to Evergreening: The term evergreening describes the efforts of brand-name companies to preserve market monopolies by making minor changes to a currently available product and pursuing a new, weaker patent; with some further review, the new patent can be invalidated by the U.S. Patent and Trademark Office. Congress has approved a process to challenge weaker patents with IPR, which is often successful because it is conducted outside of the usual court system. Brand-name drug manufacturers are lobbying to exempt pharmaceuticals from the IPR process and to block IPR challenges.
4. Pass the CREATES Act in the Senate and the FAST Generics Act in the House: These acts represent viable reforms that can stop the current actions that are impeding competition and access to lower drug costs. One example of these anticompetitive business practices is to block generic and biosimilar drug manufacturers from purchasing the brand-name drug samples they need to test and meet requirements for developing and submitting the application for a generic drug.
5. Increase Generic Utilization Among Low-Income Subsidy (LIS) Medicare Beneficiaries: There is still a surprising gap in the use of generic drugs in this subpopulation of Medicare enrollees, often lagging behind national rates of generic use. Recommendations have been made to change the LIS copay structure to incentivize the use of more generics, with the estimated potential to save more than $18 billion over the next decade.
Q: Included in your 2017 Annual Meeting agenda are Government Affairs and the Generic Drug Costs Task Force. What is the current focus of these workgroups, and are there specific areas of concern that are different than in past years?
A: Our focus is on the shared goals of both the industry and the FDA, and we look forward to seeing commitments outlined in Generic Drug User Fee Act II lead to improvements and enhancements that enable more patients to access safe and effective lower-cost generic medicines in a timely manner. Additionally, our focus is on our patient population, with drug costs posing a complex challenge for too many patients. When the conversation turns to solutions, nearly everyone agrees—generic medicines keep costs down. Generics are 89% of prescriptions, but only 27% of drug costs. We look forward to working with patients and the patient-advocate community to improve access to safe, effective, and more affordable generics and biosimilars.
Q: Your Annual Meeting also included a State Affairs session. Are there significant differences between and within states relative to generic use?
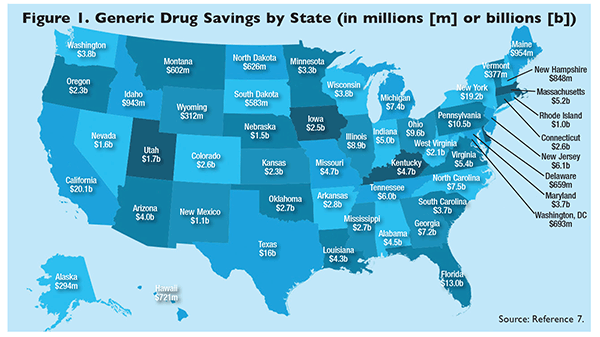
A: We refer the public to our website RX Solutions (www.gphaonline.org/issues/rx-solutions/), which contains a report demonstrating the significant differences seen across the U.S. in various generic savings reported by each state (FIGURE 1).7
Q: Whom are you recognizing this year at the Awards Recognition?
A: We selected the National Consumers League (NCL) as the recipient of our prestigious Champions of Access award. Sally Greenberg, who has served as executive director of the NCL since 2007, accepted the award on behalf of the league. Ms. Greenberg and the NCL were recognized for their work in advocating for programs and policies that promote low-cost, effective drugs that benefit patients as well as the American healthcare marketplace. The NCL [founded in 1899 as one of the first consumer organizations, with the mission to protect and promote social and economic justice for consumers and workers in the U.S. and abroad8] has long advocated for the availability of generic alternatives to brand-name treatments as a cost-saver for Americans. In the U.S., generics represent 89% of all prescriptions dispensed, but account for only 27% of total drug costs. Over the decade of 2006 to 2015, the use of generic drugs saved the U.S. healthcare system approximately $1.46 trillion. In order to reduce out-of-pocket costs while retaining the same quality of care, the NCL encourages consumers to ask healthcare providers if a generic version of their prescription is available.
REFERENCES
1. FDA. Drug Price Competition and Patent Term Restoration Act of 1984 (Hatch-Waxman Amendments). www.fda.gov/newsevents/testimony/ucm115033.htm. Accessed March 10, 2017.
2. GPhA rebrands itself and changes its name to the Association for Accessible Medicines. http://drugtopics.modernmedicine.com/drug-topics/news/gpha-rebrands-itself-and-changes-its-name-association-accessible-medicines. Accessed March 10, 2017.
3. Association for Accessible Medicines (AAM). Generic Pharmaceutical Association selects Chester “Chip” Davis, Jr. as president and CEO. www.gphaonline.org/gpha-media/press/generic-pharmaceutical-association-selects-chester. Accessed March 10, 2017.
4. AAM. About the association. www.gphaonline.org/about/the-gpha-association. Accessed March 10, 2017.
5. HHS.gov. Strategic plan: introduction. www.hhs.gov/about/strategic-plan/introduction/index.html#. Accessed March 10, 2017.
6. U.S. Department of Health and Human Services, Office of the Assistant Secretary of Program Evaluation. Understanding recent trends in generic drug prices. https://aspe.hhs.gov/sites/default/files/pdf/175071/GenericsDrugpaperr.pdf. Accessed March 10, 2017.
7. AAM. Generic drug savings and access report. www.gphaonline.org/issues/drug-costs. Accessed March 10, 2017.
8. National Consumers League. About NCL. www.nclnet.org/about_ncl. Accessed May 8, 2017.
To comment on this article, contact rdavidson@uspharmacist.com.






