US Pharm. 2011;36(3):HS-8-HS-14.
First described in the early 1700s, absence seizures have been referred to over the years as petits accès, petit mal, epilepsia minor, and pyknolepsy.1 Typically thought of as a type of childhood seizure disorder, absence seizures also may present in older populations or as a component of multiple other epileptic disorders.
Presentation
Typical absence seizures involve a transient loss of consciousness that is similar to that seen in other seizure types, but without prominent convulsive episodes. Symptoms generally are brief (usually 20 seconds); have a sudden onset that interrupts ongoing activities; and may occur up to 200 times per day, almost every day.2,3 Other manifestations may include brief unconscious behaviors (automatisms), autonomic disturbances (i.e., maintenance of heart rate, pupil function, or sweating), and brief involuntary muscle twitching (myoclonia).3 Outside factors that may trigger seizure activity include emotions, time of day, metabolic factors (including hyperventilation), and periods of changing attentiveness.1
The appearance of other symptoms is variable and differs between the various absence epilepsy syndromes. Atypical absence seizures are distinct from typical absence seizures and generally result in changes in postural tone.4 These seizures, primarily seen in Lennox-Gastaut syndrome, are outside the scope of this article.4 Absence seizures typically present during childhood—the peak age for diagnosis of childhood absence epilepsy is 5 to 7 years—but may present into early adulthood.1,2,5 Patients with childhood absence epilepsy frequently achieve remission with increasing age or treatment; however, infrequently, a patient may develop generalized tonic-clonic seizures upon entering adolescence or adulthood.1,3 Patients with a late onset of absence seizures tend to have a higher risk of convulsive seizures.1
Classification and Diagnosis
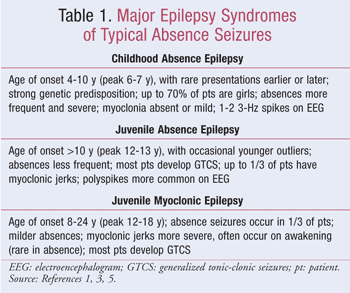
Absence seizure patients may be classified into various epilepsy syndromes defined by the Commission on Classification and Terminology of the International League Against Epilepsy (ILAE).5 Although other syndromes may be present, most patients with typical absence seizures are classified into one of three major idiopathic generalized epilepsy syndromes: childhood absence epilepsy, juvenile absence epilepsy, and juvenile myoclonic epilepsy (TABLE 1).5 While multiple syndromes occur with symptomatic absence seizures, the majority of research on the treatment of absence seizures has focused on childhood absence epilepsy.
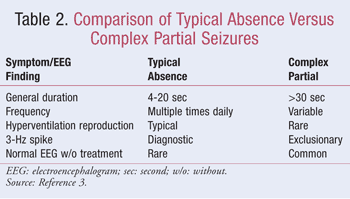
Typical absence seizures may be difficult to differentiate from complex partial seizures, although they have several distinguishing characteristics (TABLE 2).3 The major determinants of absence seizure include daily frequency, short duration (usually 20 seconds), and the ability to be reproduced by hyperventilation.1-3,5,6 Absence seizures may also be misdiagnosed as daydreaming or behavior disorders such as attention-deficit disorder.1 The electroencephalogram (EEG) is essential for differentiating epilepsy types and syndromes.
Diagnosis of absence seizure includes a video-EEG, which is best performed prior to treatment. Classic absence seizures display generalized spikes and slow-wave complexes at 3 Hz during the ictal phase.1,3,6 Usually, patients are asked to overbreathe for up to 3 minutes to trigger an absence episode; almost all untreated patients with childhood absence epilepsy will have absence activity triggered by hyperventilation.1,6 Sensitivity to light or other photic stimuli is not associated with absence seizure and has been proposed as an exclusion criterion by some researchers.1
Pathophysiology and Treatment
Absences are triggered in the thalamus when T-type calcium channels are activated, resulting in sustained-burst firing of these neurons.1,3,7 The neurotransmitter gamma-aminobutyric acid (GABA) is involved in the polarization of these channels, which potentiates the sustained-burst firing.1,3 For this reason, increased absence seizure activity occurs in patients taking GABA agonists, whereas GABA antagonists reduce the incidence of absence episodes.1,3
Historically, the treatment of absence epilepsy has been a complex issue, with significant uncertainty regarding which agent is best utilized to manage this specific manifestation. In the 1960s, recommended therapy for absence seizures ranged from anticonvulsants such as succinimides (still in use today) to an assortment of other medications, including amphetamines, chlordiazepoxide, and chloroquine.8
Since most patients prescribed an antiepileptic will remain on their selected treatment for an extended period or indefinitely, it is imperative to choose the appropriate medication.9 Most research has focused on the use of three specific antiepileptics for the treatment of absence seizures: ethosuximide, lamotrigine, and valproic acid. All are considered first-line agents by both European and ILAE guidelines.10,11
Ethosuximide: Ethosuximide (Zarontin), a succinimide, has been considered an effective agent for the treatment of absence seizures since the mid-20th century.8 Although ethosuximide’s mechanism of action is not fully understood, the blockade of a specific voltage-gated calcium channel (the T channel) in thalamic neurons appears integral to its antiepileptic activity.7 This blockade suppresses thalamic excitability, which is thought to be necessary for sustaining the characteristic spike-wave discharge of absence seizures.7 Serious adverse events linked to ethosuximide include agranulocytosis, aplastic anemia, pancytopenia, Stevens-Johnson syndrome (SJS), and systemic lupus erythematosus; however, these occur rarely and the medication is generally well tolerated.7 Standard dosing of ethosuximide is described in TABLE 3.
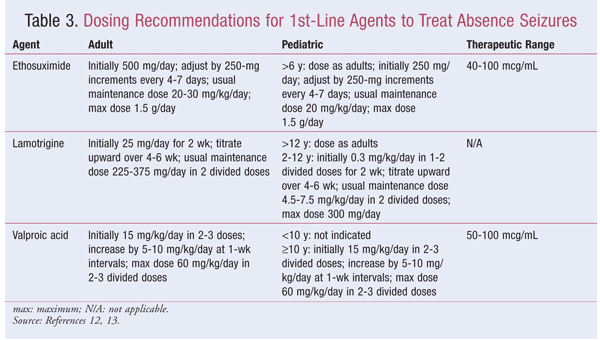
Lamotrigine: Lamotrigine (Lamictal) is thought to exert its mechanism of action through inhibition of voltage-sensitive sodium channels and subsequent glutamate release.12,13 Because of this, it may be effective in patients whose absence seizures are resistant to or inadequately controlled by valproic acid or ethosuximide.14 Serious adverse events associated with lamotrigine include angioedema, aseptic meningitis, disseminated intravascular coagulopathy, and liver failure.12,13 Of concern is the potential development of SJS, toxic epidermal necrolysis, or other serious skin rashes.15 The risk of serious skin complications can be minimized by careful titration of the medication upon initiation.15 See TABLE 3 for standard dosing. Dosage adjustments should be made for patients taking concomitant valproic acid or other CYP enzyme–inducing agents.12,13
Valproic Acid and Derivatives: The mechanism of action of valproic acid (Depakene, Depakote) remains unclear. The drug is believed to act either by increasing the concentration of GABA in the brain or by inhibiting enzymes that catabolize or block GABA reuptake.12,13 It also may inhibit voltage-sensitive sodium channels.12,13 This medication may be preferred in patients with concurrent generalized tonic-clonic or myoclonic presentations, as these seizure types respond well to treatment with valproic acid.15 Serious adverse reactions linked to valproic acid include liver failure, pancreatitis, thrombocytopenia, and hyperammonemia.12,13,15 See TABLE 3 for normal dosing. Valproic acid is pregnancy category D, so caution or avoidance in pregnant women or women of childbearing age is prudent.12,15
Agent Selection
Clinical-trial data concerning the treatment of absence seizures remains sparse. Until recently, very few prospective studies were available.16 Given this relative lack of data, the most appropriate agent for treating absence seizures remains difficult to determine. Ethosuximide and valproic acid have been shown to be equally effective as monotherapy, with control rates of more than 80%.17-19 If the onset of absence seizures occurs at an age consistent with juvenile absence epilepsy, valproic acid may be a more appropriate option, owing to the increased incidence of concomitant seizure types against which ethosuximide is ineffective.10
A study that compared lamotrigine versus valproic acid found no significant differences in seizure-free rates at 12 months.15 At the 1-month mark, however, 53% of patients taking valproic acid were seizure free, versus 5.3% of lamotrigine patients (P = .004).15 This result is likely due to the prolonged titration schedule necessary for lamotrigine (TABLE 3), which meant that, consequently, the onset of therapeutic activity for valproic acid was much quicker.15 A different study comparing these agents suggested that sodium valproate was more efficacious than lamotrigine.20 Importantly, however, the researchers were unable to reach sufficient power to determine whether their primary outcomes had been met, and patients with epileptic disorders other than absence seizures were included in the study.20
A recent large, prospective study attempted to better identify first-line therapy for absence seizures.21 A total of 453 children were randomized to ethosuximide, lamotrigine, or valproic acid monotherapy. After 16 weeks, seizure-free rates were 53%, 58%, and 29% for ethosuximide, valproic acid, and lamotrigine, respectively (P <.001).21 Additionally, attentional dysfunction was significantly more common with valproic acid than with ethosuximide (49% vs. 33%, P = .03).21 This study suggests that ethosuximide and valproic acid may be more effective than lamotrigine for treating absence seizures, with ethosuximide associated with fewer adverse effects.21
Other Therapeutic Options
Although the majority of evidence lies with the abovementioned first-line agents, a few other antiepileptics have demonstrated some degree of efficacy against absence seizures. These agents include acetazolamide, clonazepam, diazepam, felbamate, levetiracetam, topiramate, and zonisamide.14,17 In a small study, treatment with zonisamide—which has some activity at the T-type calcium channel—resulted in seizure elimination in 38% of patients with absence epilepsy, and 77% of patients had at least a 50% reduction in absence seizure activity.22 Another small study involving levetiracetam achieved freedom from seizures in 89% of absence epilepsy patients during the 6-month study period.23 While these studies do not conclude that these agents are definitive options to treat absence epilepsy, they indicate that they may be used as alternatives when primary therapies have failed.
A recent report described the use of corticosteroids in the treatment of absence seizures.24 IV methylprednisolone was attempted in a 7-year-old patient with absence seizures who failed ethosuximide, sodium valproate, lamotrigine, topiramate, and levetiracetam, as well as the ketogenic diet.24 The patient showed dramatic improvement after a 5-day course of methylprednisolone 30 mg/kg/day; she was maintained on prednisone 2 mg/kg/day twice a week for 6 weeks, and she remained seizure free for 8 months.24
Exacerbating Agents
Other antiepileptics may exacerbate absence seizures or have demonstrated little clinical efficacy. Carbamazepine, oxcarbazepine, vigabatrin, and tiagabine are contraindicated in the treatment of absence seizures.17 The GABA agonists vigabatrin and tiagabine can induce absence seizures, including absence status epilepticus.17 Carbamazepine also acts at GABAA receptors, which play a role in thalamic neuron firing, leading to an increased incidence of absence seizures.25 Other agents that should not be used are phenytoin and phenobarbital, which have the potential to exacerbate absence epilepsy, and gabapentin and pregabalin, which are ineffective.8,11,17,26
Summary
The treatment of absence seizures is a growing area of research. While absence epilepsy has not been studied as much as other types of epilepsy, recent findings are beginning to identify optimal first-line therapy. Each patient scenario should be carefully evaluated to determine the most appropriate first-line medication selection. Current data suggest that ethosuximide and valproic acid should be considered first-line agents because of their enhanced efficacy, with ethosuximide’s enhanced safety profile making it the preferred agent when no other seizure disorders are present.
REFERENCES
1. Loiseau P, Panayiotopoulos CP. Childhood absence epilepsy. International League Against Epilepsy.
www.ilae-epilepsy.org/ctf/
2. Hughes JR. Absence seizures: a review of recent reports with new concepts. Epilepsy Behav.
3. Panayiotopoulos CP. Typical absence seizures. International League Against Epilepsy.
www.ilae-epilepsy.org/ctf/ 2009;15:404-412.
4. Dulac O. Atypical absence. International League Against Epilepsy.
www.ilae-epilepsy.org/ctf/
5. Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389-399.
6. Panayiotopoulos CP. Typical absence seizures and related epileptic syndromes: assessment of current state and direction for future research. Epilepsia. 2008;49:2131-2149.
7. Huguenard JR. Block of T-type Ca(2+) channels is an important action of succinimide antiabsence drugs. Epilepsy Curr. 2002;2:49-52.
8. Auckland NL. Drugs for petit mal. Can Med Assoc J. 1965;93:707-708.
9. French JA. The role of new antiepileptic drugs. Am J Manag Care. 2001;7:S209-S214.
10. Wheless JW, Clarke DF, Arzimanoglou A, Carpenter D. Treatment of pediatric epilepsy: European expert opinion, 2007. Epileptic Disord. 2007;9:353-412.
11. Glauser T, Ben-Menachem E, Bourgeois B, et al. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006;47:1094-1120.
12. Micromedex Healthcare Series [Internet database]. Greenwood Village, CO: Thomson Reuters (Healthcare) Inc; updated periodically. Accessed December 15, 2010.
13. Lexi-Comp Online (Lexi-Drugs). Hudson, OH: Lexi-Comp, Inc; updated 2010. Accessed December 15, 2010.
14. Abou-Khalil BW. Valproate efficacy in absence seizures is hard to beat: lamotrigine comes close [commentary]. Epilepsy Curr. 2005;5:57-58.
15. Coppola G, Auricchio G, Federico R, et al. Lamotrigine versus valproic acid as first-line monotherapy in newly diagnosed typical absence seizures: an open-label, randomized, parallel-group study. Epilepsia.
16. Holmes GL, Frank LM, Sheth RD, et al. Lamotrigine monotherapy for newly diagnosed typical absence seizures in children. Epilepsy Res. 2008;82:124-132.
17. Panayiotopoulos CP. Typical absence seizures and their treatment. Arch Dis Child. 1999;81:351-355.
18. Callaghan N, O’Hare J, O’Driscoll D, et al. Comparative study of ethosuximide and sodium valproate in the treatment of typical absence seizures (petit mal). Dev Med Child Neurol. 1982;24:830-836.
19. Sato S, White BG, Penry JK, et al. Valproic acid versus ethosuximide in the treatment of absence seizures. Neurology. 1982;32:157-163.
20. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1016-1026.
21. Glauser TA, Cnaan A, Shinnar S, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010;362:790-799.
22. Kothare SV, Valencia I, Khurana DS, et al. Efficacy and tolerability of zonisamide in juvenile myoclonic epilepsy. Epileptic Disord. 2004;6:267-270.
23. Di Bonaventura C, Fattouch J, Mari F, et al. Clinical experience with levetiracetam in idiopathic generalized epilepsy according to different syndrome subtypes. Epileptic Disord. 2005;7:231-235.
24. Lichtenfeld R, Heyman E, Gandelman-Marton R, et al. Intravenous methylprednisolone pulse therapy in a young girl with intractable absence seizures. Isr Med Assoc J. 2010;12:181-182.
25. Liu L, Zheng T, Morris MJ, et al. The mechanism of carbamazepine aggravation of absence seizures. J Pharmacol Exp Ther. 2006;319:790-798.
26. French JA, Pedley TA. Initial management of epilepsy. N Engl J Med. 2008;359:166-176. 2004;45:1049-1053.
To comment on this article, contact rdavidson@uspharmacist.com.





