US Pharm. 2021;46(7):23-28.
ABSTRACT: Medication overload, another term for polypharmacy, is ubiquitous especially among older adults. It is estimated that two-thirds of those aged 65 years and older use five or more medications, including prescription, OTC, and supplements, daily. The Lown Institute published a national action plan in which it addresses the issue of medication overload and outlines five categories of interventions and 11 recommendations. Among the strategies identified in the action plan are implementing a prescription checkup, raising awareness about medication overload, improving information on deprescribing at the point of care, educating and training healthcare professionals on deprescribing, and reducing pharmaceutical-industry influence in the prescribing process.
Medication overload (MO), or polypharmacy, is defined as the use of multiple medications that pose a greater risk of harm than benefit.1 Older adults are particularly vulnerable to adverse drug events (ADEs) due to polypharmacy, altered pharmacokinetics, and cognitive decline.2
While there is no strict cutoff for the number of medications that constitute MO, it is known that as the number of medications a patient is taking increases, so does the risk of experiencing an ADE.1 Use of two, five, and seven or more medications is associated with an increased risk of ADEs of 13%, 58%, and 82%, respectively.3 In fact, for every additional medication, a person’s risk of harm increases by 7% to 10%.1
About 7,000 preventable deaths occur yearly due to ADEs.4 It is estimated that in the next 10 years ADEs will cause at least 4.6 million hospitalizations and up to 150,000 premature deaths.1 This has led to MO being called “America’s Other Drug Problem.”1 In 2014, the Department of Health and Human Services disseminated its National Action Plan for ADE prevention. Polypharmacy was identified as a potential risk factor that may lead to patient injury.2 More recently, the Lown Institute, a nonpartisan think tank that focuses on patient advocacy and equity, developed a national action plan to address the problem of MO.1
There are numerous driving factors behind MO, including a culture of “a pill for every ill,” resulting in the prescribing cascade where a medication is given to treat an adverse drug reaction; lack of knowledge on deprescribing by healthcare professionals; and a highly fragmented healthcare system in which medication-related problems and polypharmacy are common, especially during transitions of care (TOC).1,5,6
Recommendations to Eliminate Medication Overload
The Lown Institute’s Action Plan outlines 11 recommendations that are divided into five categories. These include implementation of a prescription checkup, raising awareness of MO, improving information at point of care, educating and training healthcare professionals, and reducing industry influence.1
Strategy 1: Prescription Checkups
The goal of this strategy is to eliminate or lower doses of unnecessary or harmful medications for patients who need it, in the context of an established, trusting relationship and an atmosphere free of fear for both the clinician and the patient. This is achieved using a Prescription Checkup. A Prescription Checkup is defined as a medication review focused on deprescribing. Prescription Checkups involve using evidence-based medicine and shared decision making to discontinue medications causing harm or unnecessary short-term medications that were initially used for symptom control to optimize the treatment regimen. Deprescribing can take the form of taper and discontinuation or reducing dose intensity.1
There are four main steps of a Prescription Checkup: inventory, inquiry, intervention, and follow-up. It is important to take an inventory of all prescription and OTC medications and dietary supplements. Inquiry focuses on ascertaining a patient’s values, preferences, and goals and determining how these impact medication use. During the intervention step, a plan of action for deprescribing or deintensifying drug therapy is initiated. During follow-up, the plan of action is communicated to other prescribers and the patient, and his/her caregiver is provided with a roadmap for planned interventions and an updated medication list.1 The time to benefit should also be considered when prescribing and deprescribing a medication (TABLE 1).1,7
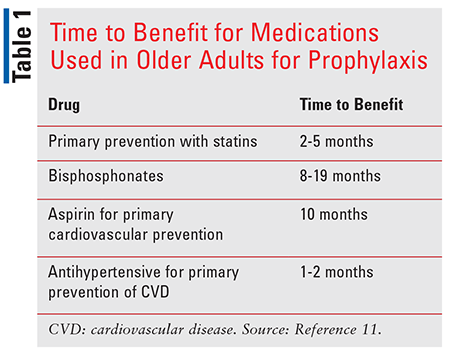
Patients who should be targeted for a Prescription Checkup include those on five or more medications, patients who have had an ADE, or those having difficulty managing their medications at home. Particular focus should be placed on moments of vulnerability, such as during TOC, the death of a loved one, sudden change in functional status, or the development of a life-threatening illness.1 The outcomes of interest for Prescription Checkups include rates of ADEs, hospitalizations, all-cause mortality, falls, medication adherence, patient-reported quality of life, and financial savings.1
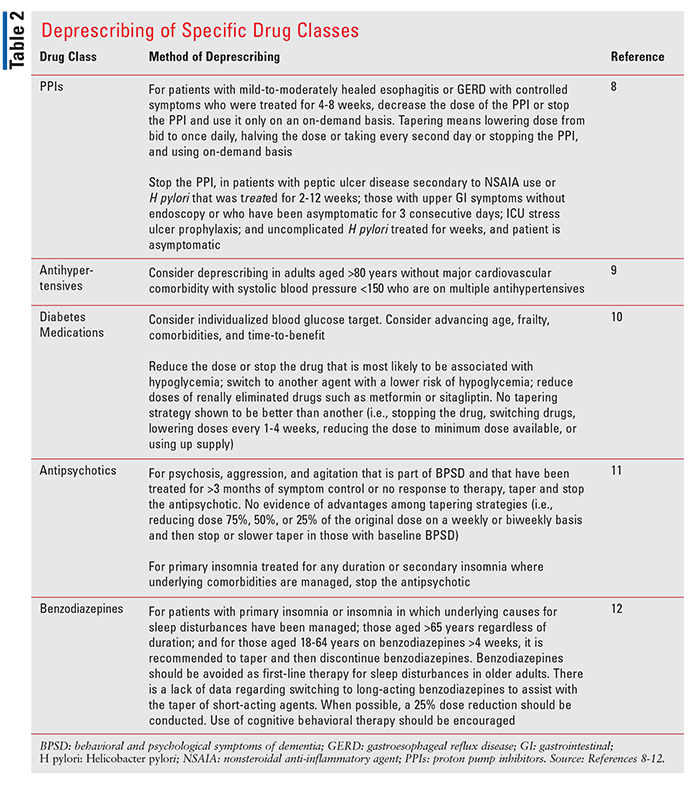
Some common classes of medications targeted for deprescribing include the proton pump inhibitors (PPIs), antihypertensives, diabetes medications, and psychotropics, in particular antipsychotics and benzodiazepines (TABLE 2).8-12 Additional medications that can be considered for deprescribing include antidepressants, medications for insomnia, analgesics, anticoagulants, antiplatelets, and anticholinergic agents.1
Strategy 2. Raise Awareness About Medication Overload
The goal of this strategy is to foster conversations between patients, families, caregivers, and clinicians on deprescribing and the avoidance of unnecessary medications. The action plan recommends creating public and patient-focused awareness campaigns on the potential harm of MO, especially as it pertains to specific medications, and creating prescriber-focused awareness campaigns to promote understanding of MO.1 The Choosing Wisely Initiative by the American Board of Internal Medicine Foundation focuses on promoting conversations between clinicians and patients by helping patients choose care that is supported by evidence, not duplicative of other tests or procedures already received, free from harm, and truly necessary. Medications are one of the services that it targets.13 A public awareness campaign on MO should address our “pill for every ill” culture of prescribing. Patients often want to stay on their current drug regimen, and providers fear causing harm by deprescribing.1
Various modes of communication should be utilized to promote this message. Written materials should be available in multiple languages, downloadable, and written at no higher than a 6th-grade reading level.14
Healthcare professionals should be educated on the scope of harm associated with MO. Enlisting the assistance of professional organizations can help to widely disseminate the message while engaging with local healthcare systems to deliver the message in a more focused approach. Academic detailing can be employed to assist with deprescribing.15,16
Strategy 3. Improve Information at the Point of Care
The goal of this strategy is to improve the availability of clear, accurate, up-to-date information on the benefits and harms of medications at the time of treatment decision making. Prescribers need unbiased information that compares the risk versus benefits of adding a new medicine, information on how long to continue therapy, advice on when it is appropriate to reduce the dose, and guidance on how to deprescribe the medication when it is no longer deemed necessary.
Low ADE reporting rates in medical records can also lead to repeated exposure to offending agents because of inaccurate discharge information and coding. Including the indications for medication use can further help pharmacists in their deprescribing efforts. The recommendations in this strategy include ensuring that clinical practice guidelines include information necessary for patient-centered prescribing; advocating crating and disseminating deprescribing guidelines; and developing a comprehensive, accurate, and timely ADE reporting system.1
The role that clinical practice guidelines play in MO also needs to be pointed out, as they often recommend treating a single condition without considering multimorbidity. Clinical practice guidelines should promote shared decision making and include information on how to deprescribe medications once no longer necessary.1
Recommendations for older adults are often based on extrapolated data from younger adults, and these gaps should be addressed. Meeting clinical practice goals may drive payment, and this could further lead to MO if a patient-specific approach to prescribing is not considered. Clinical practice guidelines that focus on deprescribing are needed for the use of statins, urinary anticholinergic drugs, antidepressants, beta-blockers and other antihypertensives, muscle relaxants, nonopioid pain medications, atypical antipsychotics, and antiplatelets.1
Although often considered a low-hanging fruit, deprescribing vitamins and minerals can be problematic. Deprescribing calcium and vitamin D, for example, may make older adults fall below the recommended dietary allowances for these nutrients.17 The NICE guidelines recommend vitamin D for the clinically extremely vulnerable, including those at very high risk of severe illness from COVID-19.18 These supplements may also be beneficial in compensating for drug-induced nutrient depletion.19
Another area that needs further re-examination is the use of automatic refills, as patients may continue to receive medications that have been deprescribed. Utilizing services such as CancelRx, an information technology tool that electronically communicates medication-discontinuation orders between electronic health records and pharmacies, may be helpful.20
Government also plays a role in facilitating deprescribing. Given that only 1% of suspected serious ADEs in the United States are reported to the FDA, one suggestion offered in the action plan is that the federal government works with the National Academies of Science, Engineering, and Medicine to address the deficiencies in our current ADE-reporting system. Closer vetting of medications that have been fast-tracked may be helpful to cut down on ADEs. Government can also enforce current regulations, since about 10% of all serious ADEs are not reported by pharmaceutical companies within the 15-day required time period.1 Educating both healthcare professionals and the public on the MedWatch program and how to report ADEs may also be beneficial.21
In 2007, the FDA launched its Sentinel Initiative, which was designed to identify potential safety issues associated with approved medications. Administrative data and insurance claims are proactively analyzed for potential signals of ADEs.22 Another initiative by the federal government is the Safe Use Initiative, which is designed to reduce preventable harm by identifying specific, preventable medication risks and developing, implementing, and evaluating cross-sector interventions with partners who are committed to safe medication use.23 Coupling these initiatives with a focus on deprescribing could help reduce ADEs.
Strategy 4: Educate and Train Health Professionals
The goal of this strategy is to educate healthcare professionals on how to analyze the potential harms and benefits of medications before prescribing them; instruct team members on how to proactively monitor for and avoid MO; and train medical personnel on how and when to pause or stop (deprescribe) medications. Recommendations to enact this strategy include enhancing health professions school curricula to teach clinical trainees proficiencies in prescribing and deprescribing and to incorporate patient-centered prescribing and deprescribing into continuing education curricula.
Clinicians should be better taught on how to evaluate drug research trials (e.g., assessing absolute risk reduction, numbers-needed-to-treat, and numbers-needed-to-harm), recognize drug-induced symptoms, perform critical thinking, engage in shared decision making, recognize the hazards of MO, and understand the process of deprescribing and of monitoring and communicating with patients following deprescribing. The COMPare Trials Project monitors outcomes of clinical trials that may have been switched to deliver a more favorable message about a drug product. Among their findings are that on average, each trial reported just 58.2% of its specified outcomes and silently added 5.3 new outcomes.24
Other areas of improvement include applying guidelines in a patient-centered approach, pairing the stage of disease with a patient’s status in life, and emphasizing nonpharmacologic interventions when appropriate.
Professional societies that offer continuing education (CE) can assist in deprescribing by including patient testimonials to help “put a face” on the ADEs associated with MO. Healthcare systems can offer Deprescribing Grand Rounds. It is important that CE programs are free of pharmaceutical-company influence. Georgetown University created the PharmedOUT program, which advances evidence-based prescribing and educates healthcare professionals and students about pharmaceutical and medical device marketing practices. PharmedOUT provides educational slideshows, videos, events, and information about CE courses free of industry sponsorship.25 The American Family Physician has online modules on deprescribing and geriatric care.26
Strategy 5. Reduce Industry Influence
The goal of this strategy is to limit pharmaceutical industry’s influence on prescribers and to limit direct-to-consumer advertising (DTCA) of medications. Recommendations to accomplish this strategy include stopping or limiting pharmaceutical industry marketing to clinicians and regulating DTCA of pharmaceuticals.1
Pharmaceutical companies spend $20 billion a year on promoting and educating healthcare professionals about their products. Companies spend an additional $6 billion a year on DTCA. This is expanding as social media is increasingly used as an advertising medium.1
DTCA often promotes costly medications while downplaying ADEs or presenting partial information about risk.27 These advertisements can encourage a pill for every ill culture as normal conditions are sometimes redefined as illnesses.1 A systematic review found that DTCA may result in patients receiving medications that were not appropriate or were not needed.28 Another concern is that although conflict of interest disclosures are required, clinical practice guidelines are often written by experts with ties to pharmaceutical industry.1
While there is no evidence that DTCA improves patient outcomes, it may increase public knowledge about diseases and treatment options.27,29 It may also lead to patients becoming more empowered in navigating their healthcare issues as well as a decrease in undertreatment of certain conditions, such as HIV.1
Given the pros and cons associated with DTCA, perhaps efforts should focus on maximizing the benefits while minimizing the risks.30-32 This may be difficult to implement since federal law does not allow the FDA to require drug companies to submit ads for approval before they are aired. The FDA only becomes involved if there are concerns about the content of the advertisements.1,33
To self-monitor, Pfizer and Bristol Myers Squibb have banned the dissemination of DTCA for 2 years after a new drug is approved in order to better assess the drug’s safety profile.1 Requiring a waiting period prior to advertising a newly approved drug is a practice recommended by the National Academies of Science, Engineering, and Medicine.1
Recently, guidelines were disseminated that can assist prescribers in evaluating patient requests that they receive as a result of DTCA. In these guidelines, the authors propose the use of the phrase “ABCD” to refer to evaluating a requested drug for ADEs, Benefits, Cost and whether the medication is appropriate for the patient’s Diagnosis.34
Studies have shown that pharmaceutical representatives can significantly influence physician prescribing, especially using samples. Healthcare systems are encouraged to limit pharmaceutical industry detailing and discourage the use of drug samples since their use often leads to the prescribing of expensive medications.35 Additional problems associated with the use of samples include that the utilization of samples bypasses screenings for drug-drug interactions and allergies, avoids an independent check by another healthcare professional, and is often associated with improper storage; up to 14% of samples have been found to include expired medications.1
Government can play a role by eliminating loopholes in the Physician Sunshine Act as this currently does not address physician use of drug samples. The Physician Payments Sunshine Act requires all pharmaceutical and medical device companies to report payments and gifts they give to doctors, including meals, research payments, consulting fees, and speaking and travel fees. However, the distribution of samples to physicians is not included in this data. This information is available to the public through the Centers for Medicare & Medicaid Services website.36 The federal government could also ensure that number-needed-to treat, effect size, absolute benefit, and number-needed-to-harm are adequately addressed in clinical trials and in DTCA.1 DTCA could also be required to indicate what percentage of the study population was aged 65 years or older, what percent had multicomorbidities, the availability of similar drugs and their ADE potential, any nonpharmacologic interventions that would produce similar or greater benefit than the advertised medication, whether there is a high risk of ADEs in older adults, and a Drug Facts Box, which would be similar to FDA’s “Nutrition Facts.”1,37 This information would need to be written by an unbiased source. Failure to meet these requirements could result in a complaint filed with the FDA and the Federal Trade Commission (FTC). The FTC ensures that advertising is truthful, nondeceptive, has claims that are backed up by evidence, and is fair.38
In lieu of an all-out ban on DTCA, federal tax breaks can be removed on DTCA. Misleading DTC advertisements should be promptly removed from circulation.39
The American Medical Student Association’s COI Scorecard developed a tool that addresses conflict of interest as it relates to healthcare policy.40 They have launched a campaign called Just Medicine, whose motto is “no kickbacks, no speakers bureau, no free samples, just medicine.”41 Doctors for America is a network of physicians and medical students who are mobilized to be leaders in putting patients over politics to improve the health of patients, communities, and the country.42 Right Care Alliance is a grassroots coalition of professional and lay persons whose focus is making healthcare institutions accountable to communities.43
Depending on the specific recommendation, it is important to get buy-in from the appropriate stakeholders. These stakeholders can include patient and family members/caregivers, clinicians, hospitals, clinics, long-term-care facilities, clinician professional societies, health professional schools and academic medical centers, government agencies and policymakers, and foundations and nonprofit organizations.1
Pharmacists can be instrumental in helping to reduce medication overload. Collaboration between all stakeholders is necessary to achieve positive outcomes.
This paper is dedicated to the author’s mother, who was and always will be her greatest supporter.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Eliminating medication overload: a national action plan. Working Group on Medication Overload. Brookline, MA: Lown Institute, 2020. https://lowninstitute.org/reports/eliminating-medication-overload-a-national-action-plan/. Accessed February 2, 2021.
2. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. (2014). National Action Plan for Adverse Drug Event Prevention. Washington, DC. https://health.gov/sites/default/files/2019-09/ADE-Action-Plan-508c.pdf. Accessed February 2, 2021.
3. Davies EA, O’Mahony MS. Adverse drug reactions in special populations - the elderly. Br J Clin Pharmacol. 2015;80(4):796-807.
4. U.S. Food and Drug Administration. Preventable adverse drug reactions: a focus on drug interactions. https://www.fda.gov/drugs/drug-interactions-labeling/preventable-adverse-drug-reactions-focus-drug-interactions. Accessed February 4, 2021.
5. Brath H, Mehta N, Savage RD, et al. What is known about preventing, detecting, and reversing prescribing cascades: a scoping review. J Am Geriatr Soc. 2018;66:2079-2085.
6. AlRasheed MM, Alhawassi TM, Alanazi A, et al. Knowledge and willingness of physicians about deprescribing among older patients: a qualitative study. Clin Interv Aging. 2018;13:1401-1408.
7. Lee SJ, Kim CM. Individualizing prevention for older adults. J Am Geriatr Soc. 2018;66(2):229-234.
8. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician. 2017;63(5):354-364.
9. Vargas F, Krishnaswami A, Goyal P. Deprescribing antihypertensives in the OPTIMISE trial. https://www.acc.org/latest-in-cardiology/articles/2020/10/05/12/58/deprescribing-antihypertensive-agents-in-the-optimise-trial. Accessed February 4, 2021.
10. Farrell B, Black C, Thompson W, et al. Deprescribing antihyperglycemic agents in older persons: evidence-based clinical practice guideline. Can Fam Physician. 2017;63(11):832-843.
11. Bjerre LM, Farrell B, Hogel M, et al. Deprescribing antipsychotics for behavioural and psychological symptoms of dementia and insomnia: evidence-based clinical practice guideline. Can Fam Physician. 2018;64(1):17-27.12. Pottie K, Thompson W, Davies S, et al. Deprescribing benzodiazepine receptor agonists: evidence-based clinical practice guideline. Can Fam Physician. 2018;64(5):339-351.
13. American Board of Internal Medicine Foundation. Choosing Wisely. https://www.choosingwisely.org/. Accessed February 8, 2021.
14. Eltorai AE, Ghanian S, Adams CA Jr, et al. Readability of patient education materials on the American Association for Surgery of Trauma website. Arch Trauma Res. 2014;3(2):e18161.
15. Ragan AP, Aikens GB, Bounthavong M, et al. Academic detailing to reduce sedative-hypnotic prescribing in older veterans. J Pharm Pract. 2021;34(2):287-294.
16. Tadrous M, Fung K, Desveaux L, et al. Effect of academic detailing on promoting appropriate prescribing of antipsychotic medication in nursing homes: a cluster randomized clinical trial. JAMA Netw Open. 2020;3(5):e205724.
17. National Institutes of Health Osteoporosis and Related Bone Diseases National Resource Center. Calcium and vitamin D: important at every age. https://www.bones.nih.gov/health-info/bone/bone-health/nutrition/calcium-and-vitamin-d-important-every-age. Accessed February 9, 2021.
18. National Institute for Health and Care Excellence (NICE). Vitamin D and clinically extremely vulnerable (CEV) guidance. https://www.gov.uk/government/publications/vitamin-d-for-vulnerable-groups/vitamin-d-and-clinically-extremely-vulnerable-cev-guidance. Accessed February 10, 2021.
19. Mospan CM. Drug-induced nutrient depletions: what pharmacists need to know. U.S. Pharm. 2019;44(12):18-24.
20. Agency for Healthcare Research and Quality. Digital Healthcare Center. CancelRx: a health IT tool to decrease medication discrepancies in the outpatient setting (Wisconsin). https://digital.ahrq.gov/ahrq-funded-projects/cancelrx-health-it-tool-decrease-medication-discrepancies-outpatient-setting. Accessed February 8, 2021.
21. FDA. Medwatch Online Voluntary Reporting Form. https://www.accessdata.fda.gov/scripts/medwatch/index.cfm. Accessed February 9, 2021.
22. FDA. FDA’s Sentinel Initiative. https://www.fda.gov/safety/fdas-sentinel-initiative. Accessed February 9, 2021.
23. FDA. Safe Use Initiative. https://www.fda.gov/drugs/drug-safety-and-availability/safe-use-initiative. Accessed February 9, 2021.
24. COMPare Trials Project. https://compare-trials.org/faq.html. Accessed February 9, 2021.
25. Georgetown University. Welcome to PharmedOut. https://sites.google.com/georgetown.edu/pharmedout/home. Accessed February 9, 2021.
26. American Family Physician. Geriatric care. https://www.aafp.org/afp/topicModules/viewTopicModule.htm?topicModuleId=55. Accessed February 9, 2021.
27. Lexchin J, Mintzes B. Direct-to-consumer advertising of prescription drugs: the evidence says No. J Public Policy Market. 2002;21(2):194-201.28. DeFrank JT, Berkman ND, Kahwati L, et al. Direct-to-consumer advertising of prescription drugs and the patient-prescriber encounter: a systematic review. Health Commun. 2020;35(6):739-746.
29. AlShammari M, Assiri G, bin Buraykan M, et al. The impact of direct-to-consumer pharmaceutical advertising on public knowledge of gastroesophageal reflux disease: a study on over-the-counter proton pump inhibitors. Patient Prefer Adherence. 2020;14:635-642.
30. Ventola CL. Direct-to-consumer pharmaceutical advertising: therapeutic or toxic? P T. 2011;36(10):669-684.
31. Shin J, Moon S. Direct-to-consumer prescription drug advertising: concerns and evidence on consumers’ benefit. J Consumer Marketing. 2005;22(7):397-403.
32. Almasi EA, Stafford RS, Kravitz RL, Mansfield PR. What are the public health effects of direct-to-consumer drug advertising? PLoS Med. 2006;3(3):e145.
33. FDA. Prescription drug advertising. https://www.fda.gov/drugs/prescription-drug-advertising/prescription-drug-advertising-questions-and-answers#control_advertisements. Accessed February 27, 2021.
34. Franquiz MJ, McGuire AL. Direct-to-consumer drug advertisement and prescribing practices: evidence review and practical guidance for clinicians. J Gen Intern Med. 2021;36(5):1390-1394.
35. Adair RF, Holmgren LR. Do drug samples influence resident prescribing behavior? A randomized trial. Am J Med. 2005;118(8):881-884.
36. Centers for Medicare and Medicaid Services. Open Payments Data. https://openpaymentsdata.cms.gov/search/physicians/by-name-and-location. Accessed February 27, 2021.
37. Dartmouth University. Inside the drug facts box. https://dartmed.dartmouth.edu/spring08/html/disc_drugs_we.php. Accessed February 27, 2021.38. Federal Trade Commission. https://www.ftc.gov/. Accessed February 27, 2021.
39. Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7(10):e1000352.
40. American Medical Students Association. AMSA Scorecard. https://www.amsa.org/scorecard/. Accessed February 27, 2021.
41. American Medical Students Association. Just Medicine. https://www.amsa.org/advocacy/just-medicine-campaign/. Accessed February 27, 2021.
42. Doctors for America. https://www.drsforamerica.org/. Accessed February 27, 2021.
43. Right Care Alliance. https://rightcarealliance.org/. Accessed February 27, 2021.






