US Pharm. 2022;47(9):22-26.
ABSTRACT: Vulvovaginal candidiasis (VVC), which is caused primarily by Candida albicans, is the second most common vaginal infection and afflicts up to 75% of women of childbearing age at least once in their lifetime. The 2016 Infectious Diseases Society of America clinical practice guideline for management of candidiasis provides recommendations for the treatment of VVC and recurrent VVC (RVVC), with therapy options including topical and systemic antifungal agents. The recent FDA approval of a new agent, oteseconazole, provides a treatment option specifically for RVVC. Effective patient education and appropriate selection of antifungal agents can help prevent the development of RVVC as well as reduce resistance patterns.
Candida, a fungus from the phylum Ascomycota, colonizes the respiratory, genitourinary, and gastrointestinal tracts of more than 30% of healthy persons during their lifetime.1 The vaginal microbiome is inhabited by bacteria of the Lactobacillus genus and by yeasts, which are known as the mycobiome. Because Candida species are the most abundant fungal organisms of the vaginal mycobiome, they can cause vaginal infections. Vulvovaginal candidiasis (VVC; commonly known as yeast infection), which is is caused primarily by Candida albicans, is the second most common vaginal infection and afflicts up to 75% of women of childbearing age at least once in their lifetime.1 Non-albicans Candida species that are known to cause VVC include C glabrata, C krusei, C parapsilosis, C africana, and C tropicalis. Recurrent VVC (RVVC), which is considered the occurrence of more than three episodes of VVC in a year, affects approximately 9% of women. Historically, RVVC has been associated with inadequate host defenses against Candida colonization; however, new research suggests a local mucosal overreaction of the immune system rather than a defective host response.1
The most common signs and symptoms of VVC are vaginal discharge, vulvar itching, burning, and pain upon urination. Symptomatic VVC is associated with elevated vaginal polymorphonuclear neutrophil (PMN) infiltration and fungal burden, which suggests that the symptoms of VVC are mediated by PMNs.1
Although RVVC does not directly correlate to mortality, disease morbidity is increasing; additionally, healthcare-associated costs are rapidly rising.1 For this reason, studies are being conducted to clarify the immunopathogenesis of RVVC and identify how to effectively treat it and prevent its recurrence.1 In patients with RVVC, factors that can alter the normal vaginal microbiome and cause Candida to thrive include changes in the hydrogen dioxide–producing Lactobacillus community and an elevated estrogen environment.
In addition to providing an overview of VVC and its management, this article will discuss oteseconazole, a recently approved medication for the treatment of RVVC, and how it compares with other treatment options.
Risk Factors for VVC
Risk factors for development of VVC include type 2 diabetes mellitus, antibiotic therapy, immunosuppressive medications, and the use of intrauterine devices and other contraceptives. Women of European, Brazilian, Egyptian, or Chinese descent and African American women are at increased risk for RVVC.1
Diagnosis
According to the 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the management of candidiasis, VVC is suspected when the patient presents with vaginal soreness, pruritus, irritation, dyspareunia, and vaginal discharge.2 Before initiation of systemic antifungal treatment, the diagnosis should be confirmed using a wet-mount preparation of saline and 10% potassium to demonstrate the presence of yeast. If the wet mount is negative for yeast, a vaginal culture should be taken.2
Treatment Overview
VVC may be treated with topical or oral antifungal agents, most commonly azoles, but these medications do not prevent recurrent infection. In some cases, patients may require prophylactic antifungal agents.1 According to the IDSA guideline, uncomplicated VVC should be treated with topical antifungals, with no single agent demonstrating superiority over the others. Topical antifungal agents that are available OTC include clotrimazole cream, 1% and 2%; miconazole cream, 2% and 4%; tioconazole ointment, 6.5%; and miconazole vaginal suppositories, 100 mg, 200 mg, and 1,200 mg. Prescription topicals include butoconazole cream, 2%; terconazole cream, 0.4% and 0.8%; and terconazole vaginal suppositories, 80 mg.3 See TABLE 1 for dosing and common adverse effects of these medications.
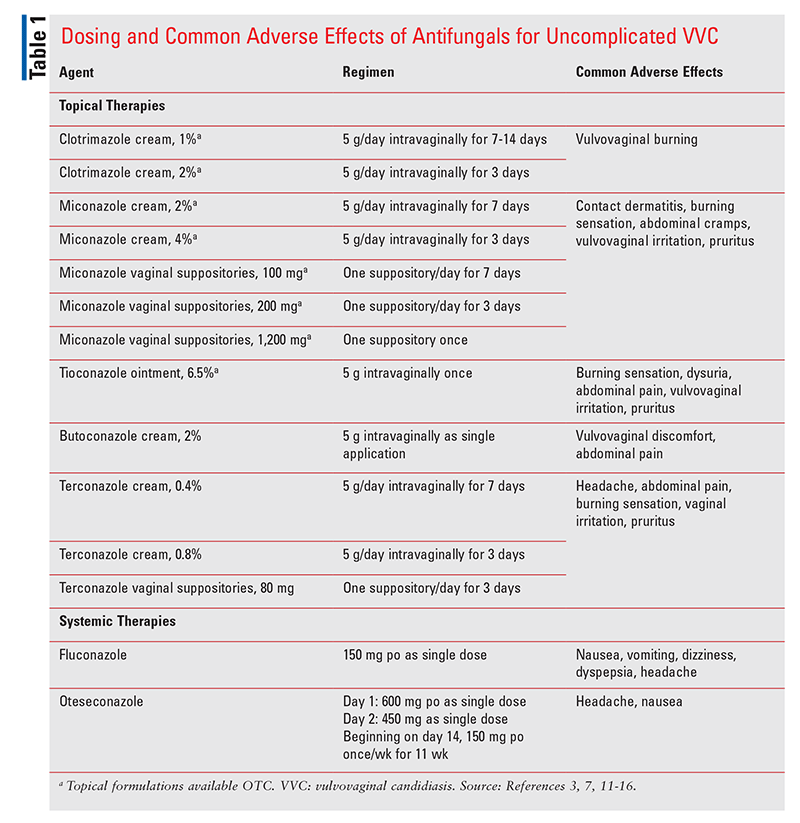
As an alternative to topical antibiotics, uncomplicated VVC may be treated with a single 150-mg dose of oral fluconazole.2 For severe acute VVC, the IDSA guideline recommends oral fluconazole 150 mg every 72 hours for a total of two to three doses. C glabrata vulvovaginitis that is unresponsive to oral azoles (e.g., fluconazole) should be treated with topical intravaginal boric acid 600 mg (in a gelatin capsule) for 14 days; nystatin intravaginal suppositories 100,000 units daily for 14 days; or topical flucytosine cream, 17%, alone or in combination with amphotericin B cream, 3%, daily for 14 days. According to the guideline, RVVC should be treated with oral fluconazole 150 mg for 10 to 14 days followed by 150 mg weekly for 6 months, which is almost double the length of time that RVVC can be treated with oteseconazole.2 Recommended preventive measures for VVC include avoiding vaginal douching, vaginal soaps, and other feminine hygiene products. Patients should be encouraged to wear cotton underwear, wipe from front to back, and change menstrual products frequently.
A retrospective chart review of premenopausal women with RVVC due to C albicans was conducted to evaluate the use of long-term fluconazole (i.e., beyond an initial 6-month course of weekly fluconazole).4 Only patients who had no risk factors for secondary VVC and who initiated a 6-month course of weekly fluconazole were included. The most common reason for additional fluconazole therapy was culture-confirmed VVC recurrence (55.1%), and the mean duration of fluconazole maintenance was 35.7 months. Resistance to fluconazole occurred in 7.5% of patients completing 6-month therapy. On follow-up, 93.6% of 51 patients reported a benefit during maintenance therapy, but 80.9% of patients relapsed after discontinuing weekly fluconazole therapy. In this study, fluconazole suppression therapy was effective in preventing VVC symptoms but was rarely curative, and VVC relapse frequently occurred after discontinuation of maintenance therapy.4 In contrast, in the ultraVIOLET study of oteseconazole, 89.7% of RVVC patients who received oteseconazole were cleared of their initial VVC infection and did not experience recurrence during the 50-week maintenance period.5
Oteseconazole
On April 28, 2022, the FDA approved oteseconazole (Vivjoa; Mycovia Pharmaceuticals) for RVVC. This new azole antifungal agent is indicated to reduce the incidence of RVVC in females who are not of reproductive potential and have a history of RVVC. Oteseconazole is an azole metalloenzyme inhibitor that targets the fungal sterol to prevent formation of the fungal cell membrane.6 Oteseconazole includes a tetrazole metal-binding group, which improves its target selectivity for fungal CYP51 and lessens its interaction with human CYP enzymes compared with other azoles. The drug’s increased fungal selectivity and profound ability to kill the fungus lessen potential side effects. In terms of its spectrum, oteseconazole has potent activity against a range of Candida species, providing excellent coverage against C albicans and C glabrata compared with fluconazole. Overall, for most Candida species, oteseconazole was an average of 40-fold more potent than fluconazole against fungus. In addition to Candida, oteseconazole has shown to be a potent inhibitor of dermatophytes including Trichophyton rubrum and Trichophyton mentagrophytes.6
The package insert for oteseconazole lists two approved dosing regimens. The oteseconazole-only regimen is as follows: On day 1, take 600 mg orally as a single dose, followed on day 2 by 450 mg orally as a single dose; then, beginning on day 14, take 150 mg orally once a week (every 7 days) for 11 weeks. The combined fluconazole-oteseconazole regimen is as follows: On days 1, 4, and 7, take fluconazole 150 mg orally; on days 14 to 20, take oteseconazole 150 mg orally once daily; then, beginning on day 28, take oteseconazole 150 mg orally once a week (every 7 days) for 11 weeks.5
Oteseconazole should be taken with food, and the capsule should be swallowed whole, not crushed, chewed, dissolved, or opened.5 This product is available in child-resistant blister packaging.5 In phase III clinical trials, the most common adverse reactions associated with oteseconazole were headache and nausea.5,8 This agent is not recommended in patients with moderate or severe (Child-Pugh class B or C) hepatic impairment, those with an estimated glomerular filtration rate of 15 to 29 mL/minute, or those with end-stage renal disease.7 Oteseconazole is contraindicated in females of reproductive potential, pregnant and lactating women, and patients with hypersensitivity to oteseconazole.5,8
To date, oteseconazole is the only FDA-approved medication for RVVC. Its approval was based on positive results from three phase III trials. Two of them (VIOLET trials) were conducted globally; the other one (ultraVIOLET trial) was based in the United States.9,10 The randomized, double-blind, placebo-controlled VIOLET trials investigated the efficacy and safety of oteseconazole for RVVC.9 Both trials comprised a 2-week fluconazole treatment period for a current VVC episode followed by 12 weeks of treatment with either oteseconazole or placebo. The primary end point was the proportion of the intent-to-treat population with one or more cultures and clinical symptoms of VVC episodes during the maintenance phase. Trial participants had three or more episodes of acute VVC in the past year, were aged 12 years or older, were capable of swallowing pills, and had a positive Gram stain test. Patients were excluded if they had a history of another vaginal condition, had poorly controlled diabetes mellitus, were pregnant, or had recently received antibiotics, antifungals, or immunosuppressive drugs. In the two trials, 93.3% and 96.1%, respectively, of women with RVVC who received oteseconazole did not have a recurrence of infection for the entire 48-week maintenance period, compared with 57.2% and 60.6%, respectively, of placebo patients.
The randomized, double-blind ultraVIOLET trial evaluated the safety and efficacy of oteseconazole versus fluconazole and placebo in patients with RVVC.10 The induction phase consisted of treating a current acute VVC episode with either fluconazole or oteseconazole. This was followed by an 11-week maintenance phase in which the participant was randomly assigned to oteseconazole or placebo. A 37-week follow-up period succeeded this maintenance period. Inclusion and exclusion criteria were the same as for the VIOLET trials. In this trial, 89.7% of patients with RVVC who received oteseconazole were cleared of their initial VVC infection and did not have a recurrence for the entire 50-week maintenance period, compared with 57.1% of patients who received fluconazole followed by placebo.
The Pharmacist’s Role
Pharmacists play a critical role in assessing patients and providing treatment recommendations. They also have the skills to collect all of the pertinent information, analyze the clinical effects, and develop a patient-centered plan. In assessments for VVC or RVVC, the patient should be screened for symptoms, history of vaginal infections, and medications previously used. Pharmacists know how to gather information to determine whether recommendation of an OTC medication versus referral to a medical provider for a prescription medication is appropriate. Patients who pick up oral antifungal agents should be screened for pregnancy, as their use can cause fetal harm. In addition, pharmacists should make an effort to counsel patients picking up creams and vaginal suppositories that their use could weaken latex condoms and diaphragms; when appropriate, these patients should also be counseled on abstinence or use of a second method of contraception. In a difference from other vaginal infections, the sexual partners of patients with VVC or RVVC do not have to be treated.
Conclusion
Based on recent trials, oteseconazole is a recommended treatment for women with RVVC who are not of reproductive potential. Based on the results of the ultraVIOLET trial, the oteseconazole-only regimen had greater efficacy in maintaining remission in RVVC. Community pharmacists can play a vital role in the prevention and management of VVC. Pharmacists should be confident in their ability to differentiate between when to treat with OTC products and when to refer to a medical provider. Patients who experience symptoms less than 2 months after using an OTC regimen or those whose symptoms do not resolve after completion of an OTC VVC regimen should seek medical attention to obtain a prescription medication. Effective patient education and appropriate selection of antifungal agents can help prevent the development of RVVC as well as reduce resistance patterns.
REFERENCES
1. Rosati D, Bruno M, Jaeger M, et al. Recurrent vulvovaginal candidiasis: an immunological perspective. Microorganisms. 2020;8(2):144.
2. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1-e50.
3. CDC. Sexually transmitted infections treatment guidelines, 2021: vulvovaginal candidiasis (VVC). www.cdc.gov/std/treatment-guidelines/candidiasis.htm. Accessed May 29, 2022.
4. Collins LM, Moore R, Sobel JD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis. 2020;24(1):48-52.
5. Mycovia Pharmaceuticals. FDA approves Mycovia Pharmaceuticals’ VIVJOATM (oteseconazole), the first and only FDA-approved medication for recurrent vulvovaginal candidiasis (chronic yeast infection). https://mycovia.com/wp-content/uploads/2022/04/FINAL-Press-Release_04.28.22.pdf. Accessed May 29, 2022.
6. Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16(18):1453-1461.
7. Oteseconazole. In: Lexi-Drugs. Hudson, OH: Lexicomp, Inc; 2022. https://online.lexi.com. Accessed June 12, 2022.
8. Vivjoa (oteseconazole) package insert. Durham, NC: Mycovia Pharmaceuticals, Inc; April 2022.
9. ClinicalTrials.gov. A study of oral oteseconazole (VT-1161) for the treatment of patients with recurrent vaginal candidiasis (yeast infection) (VIOLET). https://clinicaltrials.gov/ct2/show/NCT03561701. Accessed May 29, 2022.
10. ClinicalTrials.gov. Study of oral oteseconazole (VT-1161) for acute yeast infections in patients with recurrent yeast infections (ultraVIOLET). https://clinicaltrials.gov/ct2/show/record/NCT03840616?term=VT-1161&draw=2&rank=3. Accessed May 29, 2022.
11. Clotrimazole (topical). In: Lexi-Drugs. Hudson, OH: Lexicomp, Inc; 2022. https://online.lexi.com. Accessed July 18, 2022.
12. Miconazole (topical). In: Lexi-Drugs. Hudson, OH: Lexicomp, Inc; 2022. https://online.lexi.com. Accessed July 18, 2022.
13. Tioconazole. In: Lexi-Drugs. Hudson, OH: Lexicomp, Inc; 2022. https://online.lexi.com. Accessed July 18, 2022.
14. Butoconazole. In: Lexi-Drugs. Hudson, OH: Lexicomp, Inc; 2022. https://online.lexi.com. Accessed July 18, 2022.
15. Teroconazole. In: Lexi-Drugs. Hudson, OH: Lexicomp, Inc; 2022. https://online.lexi.com. Accessed July 18, 2022.
16. Fluconazole. In: Lexi-Drugs. Hudson, OH: Lexicomp, Inc; 2022. https://online.lexi.com. Accessed July 18, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





