US Pharm. 2023;48(2):31-36.
ABSTRACT: The continual advances associated with personalized medicine, also known as precision or individualized medicine, have the potential to aid clinicians in understanding how an individual’s genetic makeup can influence and guide decisions with regard to the diagnosis, treatment, and prevention of certain medical conditions. This valuable information provides a more thorough understanding of the impact of how one’s genes, environment, and lifestyle factors affect certain diseases. This is of particular importance for rare and/or difficult-to-treat diseases, as well as those without FDA-approved therapies. One of the fastest-growing areas in personalized medicine is the field of oncology, and research is exploring other areas for personalized medicine to diagnose and treat other diseases. Examples of personalized medicine include the targeted therapies indicated for the treatment of human epidermal growth factor receptor 2–positive breast cancer and tumor marker testing to diagnose cancer. Other examples of recent approvals of personalized medicines include indications for treatment of familial hypercholesterolemia and treatment of generalized myasthenia gravis.
The National Institutes of Health’s National Human Genome Research Institute (NHGRI) indicates that the majority of medical treatments are designed for the general population with a “one size fits all” approach, and while many of these therapies are effective, not all patients may respond to them.1 According to the NHGRI, personalized medicine, also referred to as precision medicine, is a promising and evolving practice of medicine that employs an individual’s genetic profile, which can guide clinicians in ascertaining the best approaches to possibly prevent, diagnose, and treat and/or manage certain diseases and medical conditions.2 Additionally, using a patient’s genetic profile can be valuable in prescribing the best medication or therapy and dosage to suit the individual needs of the patient.1-3 The Personalized Medicine Coalition (PMC) indicates that personalized medicine has the potential to enhance treatment outcomes across a spectrum of diseases via targeted therapies, therefore adding to the effectiveness of medicine. In a recent report from the PMC, health experts note that personalized medicines now account for more than one of every four drugs the FDA has approved since 2014.4,5 In general, the goals of personalized/precision medication are to align the treatment with the unique patient profile.1,3
Health experts indicate that through the merging of pharmacology and genomics, pharmacogenomics studies provide valuable information that aids clinicians in understanding the impact of an individual’s genomic fingerprint and how that person responds to a particular therapy. Moreover, this method aims to improve the patient’s drug response and diminish treatment adverse effects by matching the ideal drug and dosage established by a patient’s genetic makeup.6 The NHGRI also notes that ongoing development and accumulation of data for personalized medicine continues to expand through the Human Genome Project (HGP), which was executed from 1993 to 2003 and is a landmark global scientific research effort designed to create the first sequence of the human genome and that of several additional well-studied organisms.2,3 The HGP and thousands of follow-on studies are providing researchers with an opportunity to develop gene-targeted treatments.2,3 More in-depth information about the HGP can be found at www.genome.gov/about-genomics/educational-resources/fact-sheets/human-genome-project.
The FDA indicates that individualized therapeutics (personalized therapies) are products that are aimed at addressing the unmet needs of patients who do not respond to traditional therapies.3 Moreover, these unique therapies are becoming progressively more practical and can be incorporated into clinical practice because of expanded knowledge about individual variability (e.g., genetic and lifestyle treatments), which may enable clinicians to tailor dosages to the needs of individual patients.
Precision medicines have enabled clinicians to gain a better understanding regarding new rare genetic diseases because of the use of next generation sequencing (NGS) technologies.3 NGS technologies are key to the integration of precision medicine and are adept at swiftly recognizing or “sequencing” large sections of an individual’s genome.7 Diagnostic technologies are also integral components of personalized medicine since these types of tests can assist prescribers in selecting a medical treatment that is best for each patient.1,2 For example, clinicians will sometimes perform molecular testing in the care of some patients with cancer to ascertain information to select treatments that may enhance rates of survival and minimize treatment-related adverse effects.7
However, the FDA notes that while personalized therapies can be promising, as with other treatments they are also associated with various challenges including: recognizing the mechanisms that contribute to the manifestations of adverse effects (for example what causes an individual’s body to develop a rash or have nausea and/or vomiting) and understanding the variances in the efficacy of a treatment from one individual to another.3
In an effort to utilize and expand the clinical benefits associated with personalized medicine, the FDA is continually investigating the advancements in the use of innovative technology and created a cloud-based portal, known as precisionFDA, for global community research and development that enables researchers to share data and tools to test, pilot, and validate existing and new bioinformatics approaches to NGS processing.3 More information about this platform can be found at https://precision.fda.gov.
The FDA indicates that targeted therapies linked with personalized medicine represent a significant progression in drug development, and the field of oncology is one of the most rapidly expanding areas of therapeutics for certain types of cancers.7,8 The two types of treatments often employed in personalized medicine in cancer care include targeted therapies and immunotherapies.9
According to the American Cancer Society, personalized medicine in the care of cancer patients provides the following benefits9:
• Recognizing those at greater risk for cancer
• Detecting cancer early
• Obtaining accurate diagnosis
• Selecting optimal cancer therapy
• Assessing patient response to therapy.
Innovations in Personalized Medicine
The FDA is creating and assessing animal models to evaluate safety and effectiveness of bacteriophage cocktails for the treatment of bacterial infections that are classified as antibacterial resistant.3 Other innovations involve development and usage of gene therapies and pharmacogenetic tests. Pharmacogenetic tests provide clinicians with information regarding how an individual patient’s genetic makeup impacts the patient’s therapeutic response to certain treatments.3 In addition, FDA scientists are conducting research aimed at essential regulatory science inquiries that are related to drug approval regarding the usage of immunotherapy agents and other novel agents that can be employed for the treatment of various cancers. They are also exploring how genetics plays a role in the development of immune-related adverse effects and therapeutic response to these agents.3
In January 2021, the FDA announced its actions in providing clinicians with information on the development of novel therapies as the role of personalized medicine continues to expand. The FDA issued a draft guidance on submissions for investigational new drugs for individualized antisense oligonucleotide (ASO) agents that are intended to treat a severely debilitating or life-threatening genetic disease.10,11 The guidance was established to instruct those sponsors developing ASO products on a methodology to interacting with and making regulatory submissions to the FDA. The guidance addresses the following points10,11: the method to obtaining response from the FDA with an established communication plan; the expectations and procedure for creating regulatory submissions to the FDA; and recommendations about the condition for Institutional Review Board review of the protocols within and how to acquire informed consent.
Ongoing Research Efforts
Ongoing research efforts continue to explore the value of personalized medicine. The FDA’s Division of Translational and Precision Medicine (DTPM) is a team of translational scientists with expertise in clinical pharmacology, human genomics, epidemiology, and molecular biology.12 In general, its goals include confirming that clinical pharmacology principles and precision medicine strategies are utilized properly in all phases of drug development to expand benefit and diminish risk to patients.12 The DTPM notes that the goals of precision medicine include progressing the expansion and accessibility of safe and effective targeted treatments while also fostering scientific research efforts to integrate omics-based tools, technologies, and biomarkers in drug development.12 The DTPM focuses on the incorporation of genomics, advancement of targeted therapies, and support of biomarker qualification across therapeutic areas, and they execute these applications via their fundamental operational functions, which include the following12:
Regulatory Review: In the assessment of intrinsic and extrinsic factors on drug exposure, safety, and efficacy and to assist and hasten the advancement of drugs and biological products, DTPM reviewers employ pharmacogenomic, pharmacokinetic, and pharmacodynamic principles.
Regulatory Science: DTPM performs research projects and works with researchers throughout government, academia, and industry to identify and address knowledge gaps. To enhance patient care, the researchers concentrate on approaches to expand effectiveness in drug development by utilizing biomarkers, genomics, and pharmacokinetic/pharmacodynamic data.
Guidance and Policy Development: DTPM acts to recognize evolving issues in drug development to inform stakeholders on FDA’s current philosophy through guidance and policy development.
Education and Outreach: Via public workshops, conferences, and other partnerships, DTPM spreads awareness of drug discovery and development with the community.
In a 2021 report published by the PMC, health experts revealed the following achievements with regard to personalized medicine13:
Personalized medicines accounted for more than one-third of new drug approvals for 4 of the past 5 years. In 2021, of the 48 new therapeutic molecular therapies approved by the FDA’s Center for Drug Evaluation and Research, 17 were approved as personalized medicines, which represented an estimated 35% of all newly approved therapeutic molecular entities (see TABLE 1).
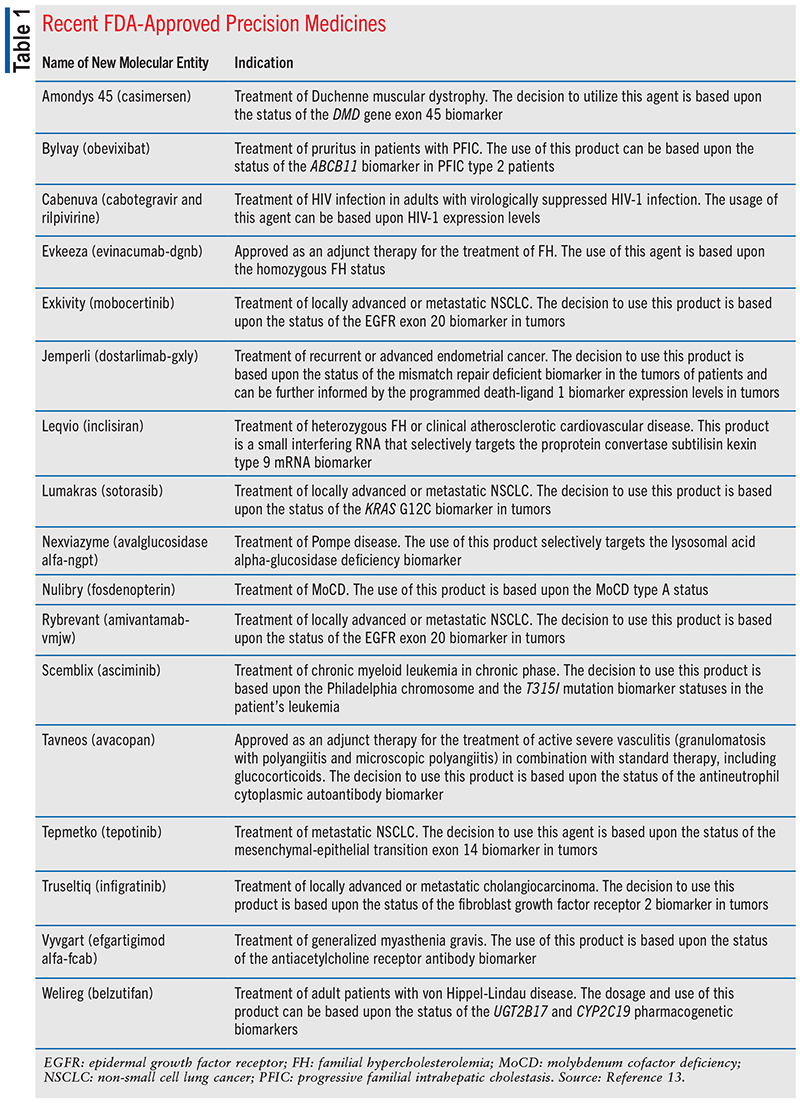
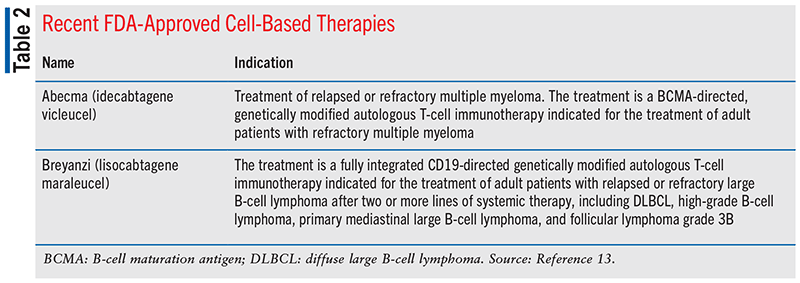
According to the report, in 2021, FDA’s Center for Biologics Evaluation and Research also expanded the indications for some existing personalized therapies and approved two novel cell-based immunotherapies for cancers that are often challenging to treat (see TABLE 2). The approval of these novel agents is quite remarkable since these personalized therapies involve the transplantation of normal genes into a patient’s own cells to alter specific cellular functions. There are currently eight cell-based or gene therapies approved by the FDA.13
The FDA also granted recognition of the first tumor mutation database, thus permitting test developers to employ real-world data to support the clinical validity of new diagnostic tests, and approved some new diagnostic indications that will enable clinicians to make decisions about targeted therapies for various health conditions.13
Recent News and Clinical Data
Findings from a study published in the International Journal of Molecular Sciences indicated that precision medicine may be essential in the treatment of melanoma in those who do not respond to traditional therapies or experience severe adverse events.14 The authors indicated that precision medicine has enabled clinicians to recognize and understand the numerous processes involved in development of cancer, thereby improving outcomes. Moreover, advancements have led to the development of new pharmacologic targets and potential biomarkers used to predict the response or adverse events to treatments.14
A study published in JCO Precision Oncology indicated that personalized medicine presents innovative opportunities to treat patients with cancer. In this study, researchers assessed the impact of various clinical practice gaps associated with diagnostic testing-informed personalized medicine strategies in the treatment of advanced non-small cell lung cancer (aNSCLC) and revealed that an estimated 64% of potentially eligible patients with aNSCLC are not benefiting from precision oncology therapies appropriate for their disease. The authors concluded that these findings are possibly indicative of comparable gaps in other cancer types. They also noted that there is a need for an expansion of knowledge with regard to implementing strategies to improve the integration of precision oncology into clinical practice and to understand the clinical benefits associated with personalized medicine.15
In another publication in JCO Precision Oncology, researchers identified 26 unique genetic variants in African American men that appear to be associated with more severe prostate cancer in this population group. The authors noted that further examination of these genetic associations will provide greater awareness in understanding the increased risk of prostate cancer in African American men, potentially leading to earlier diagnosis and in diminishing current health disparities.16
In November 2022, Cancer Research UK, the University of Manchester, and Roche announced the launch of a multidrug, precision medicine DETERMINE trial, which seeks to ascertain if available agents, including those that are licensed for more common types of cancer, could also benefit patients with rare cancer types that the drug is not currently licensed for.17 The trial is recruiting both adult and pediatric patients with rare cancers. More information can be found at www.cancerresearchuk.org/funding-for-researchers/our-research-infrastructure/our-centre-for-drug-development/determine-overview.
Recently, the PMC published a report entitled A Strategic Plan for Advancing Personalized Medicine in 2022. The report indicates that the PMC will explore the growing role of personalized medicine across multiple disease states. The PMC will continue to address emerging matters with regard to regulation, reimbursement, and the integration of precision medicine into healthcare systems. Its efforts will also focus on expanding the use of personalized medicine.18
In February 2022, the PMC published a white paper entitled Using Health Data to Advance Personalized Medicine: Challenges and a Path Forward, which discusses data-related opportunities and barriers directly affecting the delivery of healthcare. The report discusses three key topics that will impact the adoption of personalized medicine into healthcare, including how electronic healthcare data are used, protecting patient privacy, and how real-world clinical data are utilized.4
The Role of the Pharmacist
As the integration of personalized medicine into clinical practice continues to expand, it is imperative that pharmacists remain updated on advancements in the promising roles in the diagnosis, treatment, and prevention of certain diseases, recent approvals, and ongoing research. Published literature indicates that pharmacists can be instrumental in expanding awareness about pharmacogenomics and personalized medicine due to their drug expertise in pharmacokinetics and pharmacodynamics.19 For example, the American Society of Health-System Pharmacists (ASHP) indicates that pharmacogenomic testing can improve medication-related outcomes across the continuum of care in all health system practice settings.20 AHSP states that benefits may include reduction in suboptimal clinical outcomes, decreased cost of treatment, better medication adherence, more suitable selection of therapeutic agents, reduced duration of treatment, and enhanced patient safety through pharmacist intervention.20 Moreover, due to their expertise, pharmacists can be instrumental in working in collaboration with clinicians by ensuring optimal drug selection and drug dosing based on results from pharmacogenomic testing.20
Conclusion
The ongoing advancements in the field of personalized medicine and the approval of personalized medications continue to create the opportunity to improve clinical outcomes for many patients while also making healthcare more efficient and effective. Additionally, the field of personalized medicine has the potential to identify and address the underlying molecular mechanisms responsible for the development of certain diseases. The integration of personalized medicine provides clinicians with valuable tools that can be instrumental in the diagnosis, treatment, and prevention of diseases. Due to their drug expertise, pharmacists are well poised to educate both prescribers and patients about the core value of personalized medicine, especially with regard to the use of pharmacogenomics in the delivery of individualized healthcare to aid in selecting optimal treatments. They can also provide information about the efficacy and safety data of FDA-approved precision medicines and those under investigation as well as the clinical benefits associated with personalized medicine.
REFERENCES
1. NIH. Personalized medicine. May 10, 2022. www.genome.gov/genetics-glossary/Personalized-Medicine. Accessed November 29, 2022.2. NIH. Human Genome Project. August 24, 2022. www.genome.gov/about-genomics/educational-resources/fact-sheets/human-genome-project. Accessed November 29, 2022.
3. FDA. Focus area: individualized therapeutics and precision medicine. September 6, 2022. www.fda.gov/science-research/focus-areas-regulatory-science-report/focus-area-individualized-therapeutics-and-precision-medicine. Accessed November 29, 2022.4. Personalized Medicine Coalition. Using health data to advance personalized medicine: challenges and a path forward. February 17, 2022. www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/Using_Health_Data_to_Advance_Personalized_Medicine_Challenges_and_a_Path_Forward.pdf. Accessed December 2, 2022.
5. Personalized Medicine Coalition. Personalized medicine at FDA: the scope & significance of progress in 2019. Published February 21, 2020. www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/PM_at_FDA_The_Scope_and_Significance_of_Progress_in_2019.pdf. Accessed December 2, 2022.6. Healio. What is precision medicine? www.healio.com/hematology-oncology/learn-genomics/course-introduction/what-is-precision-medicine. Accessed December 2, 2022.
7. FDA. Precision medicine. September 27, 2018. www.fda.gov/medical-devices/in-vitro-diagnostics/precision-medicine. Accessed November 29, 2022.8. Quinn C, Young, C, Thomas, J, et al. Estimating the clinical pipeline of cell and gene therapies and their potential economic impact on the US healthcare system. Value Health. 2019;22(6):621-626.
9. American Cancer Society. Precision or personalized medicine. What is precision medicine? July 14, 2022. www.cancer.org/treatment/treatments-and-side-effects/treatment-types/precision-medicine.html. Accessed November 30, 2022.10. FDA. FDA takes steps to provide clarity on developing new drug products in the age of individualized medicine. January 4, 2021. www.fda.gov/news-events/press-announcements/fda-takes-steps-provide-clarity-developing-new-drug-products-age-individualized-medicine. Accessed November 29, 2022.
11. FDA. IND submissions for individualized antisense oligonucleotide drug products: administrative and procedural recommendations guidance for sponsor-investigators. January 2021. www.fda.gov/regulatory-information/search-fda-guidance-documents/ind-submissions-individualized-antisense-oligonucleotide-drug-products-administrative-and-procedural. Accessed November 29, 2022.12. FDA. Division of Translational and Precision Medicine (DTPM). February 2, 2021. www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/division-translational-and-precision-medicine-dtpm. Accessed November 29, 2022.
13. Personalized Medicine Coalition. Personalized medicine at the FDA: the scope & significance of progress in 2021. https://personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/Personalized_Medicine_at_FDA_The_Scope_Significance_of_Progress_in_2021.pdf. Accessed January 10, 2023.14. Valenti F, Falcone I, Ungania S, et al. Precision medicine and melanoma: multi-omics approaches to monitoring the immunotherapy response. Int J Mol Sci. 2021;22(8):3837.
15. Sadik H, Pritchard D, Keeling DM, et al. Impact of clinical practice gaps on the implementation of personalized medicine in advanced non-small-cell lung cancer. JCO Precis Oncol. 2022;10(6):e2200246.16. Trendowski MR, Sample C, Baird T, et al. Germline variants in DNA damage repair genes and HOXB13 among black patients with early-onset prostate cancer. JCO Precis Oncol. 2022;11(6):e2200460.
17. University of Manchester. Precision medicine trial opens for rare adult and paediatric cancers. November 23, 2022. www.manchester.ac.uk/discover/news/precision-medicine-trial-opens-for-rare-adult-and-paedatric-cancers/. Accessed December 1, 2022.18. Personalized Medicine Coalition. A strategic plan for advancing personalized medicine in 2022. January 2022. www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/Strategic_Plan_20224.pdf. Accessed December 2, 2022.
19. Elewa H, Awaisu A. Pharmacogenomics in pharmacy practice: current perspectives. Integr Pharm Res Pract. 2019;11(8):97-104.20. ASHP statement on the pharmacist’s role in clinical pharmacogenomics. Am J Health Syst Pharm. 2015;72(7):579-581.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






