US Pharm. 2008;33(3):HS2-HS8.
Each year, millions of infants
and children require sedation and pain control for medical procedures. Over
the past 25 years, great strides have been made to increase patient safety,
including systematic reviews of adverse event (AE) prevalence and the
institution of guidelines endorsed by several groups, including the American
Academy of Pediatrics (AAP), the American Academy of Pediatric Dentistry
(AAPD), and the American Society of Anesthesiologists (ASA). It has been
observed that children younger than 6 years or those with developmental delay
may require deeper sedation than other patient populations.1 The
continual struggle faced by practitioners is achieving "successful sedation"
while preventing or avoiding AEs. Previous reports have demonstrated the
incidence of "failed sedation" to be anywhere from 0.2% to 50% of patients,
although more recent reviews have shown this percentage to exist closer to the
lower end of the range.2-5 However, the likely incidence of serious
AEs resulting from pediatric sedation is less than 1 per 10,000.6
The actual incidence is difficult to determine due to volunteer reporting of
events, which greatly decreases the validity of reported numbers. Any trial
looking at AEs would require a large number of subjects to accurately assess.
In the mid 1980s, guidelines
were created by several organizations with the intention to reduce AEs,
specifically severe events such as permanent neurological injury and death.
7 These guidelines have been systematically updated and the reported
incidence of morbidity from sedation has been reduced from 1 per 10,000 to 1
per 60,000.8 Even with the advent of continually updated
guidelines, AEs are still a concern for any practitioner. This review will
serve to identify and explain proposed theories that describe why AEs happen
and how current guidelines reduce the risk in pediatric moderate sedation.
Definition of Sedation
Current guidelines,
spearheaded by the AAPD and AAP, define sedation one of four ways: minimal
sedation (anxiolysis), moderate (procedural or conscious) sedation, deep
sedation, or general anesthesia.9,10 Moderate sedation is defined
as a pharmacologically induced state that allows patients to tolerate painful
procedures while maintaining protective reflexes (i.e., gag reflex and cough)
and adequate airway control.5 Moderate sedation is the most
commonly used form of sedation outside of the operating room and is frequently
used in procedures such as MRI, CT, and dental work.
Prevalence of Adverse
Events
There exist no
multicentered, accurately powered trials to detect the frequency of AEs
associated with sedation in pediatric patients. Data from several individual,
retrospective studies indicate that the incidence of overall AEs due to
pediatric sedation ranges from 0.6% to 25%5,8,11; however, these
analyses have also found that close to 99% of events are considered mild to
moderate in nature. The most common AE in pediatric sedation is hypoxia.8
The wide gap of reported events is likely due to inconsistency with several
factors, including how trials define "complication" (e.g., O2
sat ?80% vs. ?90%) or "successful sedation," as well as the particular drugs
used or studied. The newly formed Pediatric Sedation Research Consortium
(PSRC), comprised of 35 institutions, is dedicated to improving sedation and
anesthesia practices via a database where individual events can be shared by
participating institutions.8 During the past four years, the PSRC
has compiled over 30,000 encounters. The top three drugs utilized were
propofol (50.1%), midazolam (27.1%), and ketamine (13.6%).8 One
thousand and twenty events have been recorded (3.4%) since the launching of
the PSRC, which is .336 of these events (33%) required some type of
intervention, but only two (.2%) were considered serious (zero death, one
cardiac arrest, one aspiration). According to the PSRC, one in 1,500 sedations
will result in an event requiring unplanned admission. Events are reported to
the PSRC voluntarily and are likely underreported. In terms of AEs based on
age, Malviya et al found, in a study of 1,140 patients, that neonates
experience significantly more events than 1- to 12-month-old infants (P
=.04), 13- to 24-month-old children (P =.005), and 25-month- to
12-year-old children (P <.0001). They also found that infants 1 to 12
months of age experience more events versus 25-month- to 12-year-old children (
P =.0001).11 While comparing intravenous fentanyl and midazolam
to intravenous ketamine, midazolam, and atropine, Pitetti et al found that
patients in both groups who experienced an AE were likely to be older (7.4 y
vs. 6.6 y, P =.02).5
Causes of Pediatric Adverse
Events
Compared to adults,
pediatric patients, in terms of moderate sedation, pose the highest risk and
offer the lowest tolerance for error. In many cases, they need deeper sedation
than adults, and this may contribute to the use of increased doses or
polypharmacotherapy. Past studies have tried to identify possible mechanisms
that may cause a patient to be at higher risk for developing an AE during
sedation for outpatient procedures. Cote et al looked at 95 case reports
compiled nationally, all defined as either death or permanent neurological
injury.7 The authors found that 80% of these events presented
initially as respiratory compromise, which has been previously described as
the most common of AEs in pediatric sedation. Cote et al attempted to
determine why some patients deteriorate to the point of needing emergency
rescue care while undergoing sedation. The analysis by Cote et al posed seven
possible causes of serious AEs due to sedation: 1) drug interactions; 2) high
dose or drug overdose; 3) premature discharge; 4) prescription/transcription
errors; 5) inadequate understanding of administered medications
(pharmacokinetics/pharmacodynamics; 6) administration by unsupervised
technicians; and 7) administration by parents or family member.12
The group also postulated that several events may have been due to multiple
categories. Data are conflicting regarding whether certain drug classes are
responsible for a larger number of events than others. The report by Cote et
al found that there was no correlation between drug class or route of
administration and events. Pitetti et al looked at 1,244 events occurring in
1,215 patients and found no difference between age, sex, race, ASA class
(defined by level of systemic disease experienced by the patient), use of
premedication, or level of consciousness achieved during sedation.5
These patients were only sedated with varying combinations of five drugs.
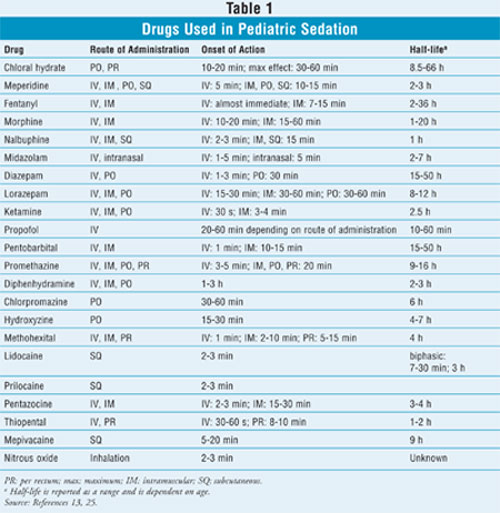
Drug Interactions:
Cote et al found drug interactions to be associated with the highest
incidence of adverse events.7 An analysis of 95 case reports found
that there was a marked increased risk of events when three or more
medications were used for sedation (18/20 patients experienced event vs. 7/70;
P =.0006). Obtaining an accurate pharmacy history through medication
reconciliation is a vital part of the health evaluation before sedation is
initiated. Drug interactions are a continual concern, specifically drugs that
interfere with hepatic metabolism of sedatives (ciprofloxacin, fluconazole,
fluoxetine, amiodarone, antiretrovirals),13 medications causing
delayed renal clearance of medications, and drugs that may cause synergistic
effects. In addition, herbal medications such as St. John's wort may interfere
with the CYP450 system, echinacea may prolong certain drug effects, and kava
has the potential to increase the effects of sedatives. Nitrous oxide is
commonly used in dental settings and, if diluted and used correctly, is very
safe and efficacious; however, when combined with other sedatives, it can
produce levels of deep sedation that the administrator/practitioner may not
have anticipated.14 Local anesthetics (LAs), when used in
combination with opioids, can cause a decrease in protein binding of the LA
and increase arterial CO2, both of which could precipitate seizure
activity.1
High Dose/Drug Overdose:
Drug overdose is defined as greater than or equal to one drug administered in
a dose greater than 1.25 times the maximum recommended dose.12 It
has been shown that 71% of sedative-overdosed patients have suffered AEs.7
Pitetti et al found that patients given higher doses of opiates and
benzodiazepines were more likely to experience an event (fentanyl 3.0 mcg/kg
vs. 2.6 mcg/kg, P =.001; midazolam 0.13 mg/kg vs. 0.11 mg/kg, P
=.01). They also found that patients receiving higher doses of midazolam in
combination with morphine were more likely to experience events (0.27 mg/kg
vs. 0.15 mg/kg, P =.03). The most common event that occurred in this
study was vomiting (77%).5 Drug overdoses are recognized as the
second most common cause of AEs in sedation; however, none of the reported
cases involved a 10-fold increase in dose.7 This suggests a lack of
knowledge of administered drugs, as the errors were not decimal in nature
Premature
Discharge/Inadequate Understanding of Administered Drug:
Practitioners should be familiar with the pharmacokinetics and
pharmacodynamics properties of sedation agents. Certain drugs used in sedation
(chloral hydrate, meperidine, pentobarbital, phenothiazines) have extended
half-lives compared to other sedative drugs, and patients may require longer
observation times so that the effects can completely wear off.13
Direct pharmacy involvement has been recognized to decrease medication errors,
specifically in the areas of pharmacokinetics.16 Patient age can
affect drug half-life; chloral hydrate has a half-life of approximately 28
hours in newborns and 10 hours in infants.17 Promethazine has vast
differences in half-life between infants and adults (7.1 h vs. 20 h). Another
consideration is intramuscular administration, as depot effects can
significantly alter pharmacokinetics. Patient discharge prior to complete drug
metabolism can be dangerous if prolonged effects persist after the patient has
been discharged home. Depending on the half-life and time to secondary effect,
patients may not have immediate access to resuscitative care
Prescription/Transcription Error:
Cote et al found that it was not individual drugs that specifically caused
events but rather a failure to follow procedure. Improper combinations,
medication errors, faulty dosing, and inadequate monitoring were all targeted
as possible systems breakdowns resulting in AEs. Direct pharmacy involvement
may play a vital role in eliminating these errors, specifically in terms of
mistranscription (tsp vs. tbsp) or drug calculations (mg/kg)
Administration by
Unsupervised Technician/Parents/Family Member:
Administration of drugs in an outpatient setting, either at a
physician/dentist office or, more dangerously, at home by the family, has been
an ongoing problem in relation to pediatric sedation. More deaths and
neurological injuries have occurred in the outpatient than the inpatient
setting (deaths: 82.1% vs. 30.2%, P <.001; neurological injury: 10.7%
vs. 7.0%, P <.001).12 Lack of pulse oximetry plays a
vital role in these statistics. In terms of predicting life-threatening
events, pulse oximetry is by far the most important monitoring device.18
Even in a small subset of patients, better outcomes have been observed when
some type of monitoring is available (14 events vs. 4, P <.001).
Outpatient sedation leaves the potential for improper resuscitation due to
inadequate equipment if a respiratory event should occur. When comparing
inadequate resuscitation rates between inpatients and outpatients, only 2.3%
of inpatients versus 57.1% of outpatients (P <.001) received inadequate
resuscitation.12 Yet another risk factor for adverse sedation
events is the coexistence of underlying disease states. Malviya et al found
that children with ASA physical status III and IV experienced more events
compared to children meeting ASA criteria for status I and II (P
<.0001) and were more likely to have respiratory complications (P
<.0001).11 ASA criteria evaluate patients based on severity of
comorbid conditions and may help administrators identify those at higher risk
for AEs. Some conditions may place a patient in a higher ASA class.
Prevention of Adverse Events
Direct pharmacy involvement,
specifically having a trained pharmacy specialist present during sedation,
could effectively help to decrease several of the identified causes of AEs.
Drug interactions and drug overdoses can be minimized by providing an accurate
patient medication history and properly examining drugs both before and while
they are being administered to the patient. Accurate knowledge of drug
pharmacokinetics could decrease the likelihood of a patient being discharged
before a fully recovery is achieved. A systematic approach to medication
administration should be utilized to eliminate transcription errors.
Calculating mg/kg limits, using standard dosing regimens, and double- or
triple-checking doses should be employed. Drs. Charles Cote and Theodore
Striker developed the first set of pediatric sedation guidelines in 1983 after
three deaths that occurred at one dental practice.19 The original
guidelines were endorsed by the AAP, and over the past 25 years they have been
updated numerous times, most recently in 2006 in conjunction with both the
AAPD and the ASA. Several other organizations have also published guidelines
on how to manage sedation in patients in a variety of settings. These
guidelines have helped to properly define different levels of sedation as well
as to provide recommendations for better administration of sedative drugs and
monitoring both during and after procedures are performed. New requirements
allow only properly trained, licensed practitioners to administer sedative
medications. These practitioners, who at a minimum should be able to provide
bag-mask-valve ventilation to oxygenate a patient, should be accompanied by
other clinical staff who are all well versed in emergency protocols to
minimize serious AEs from occurring.14,20 Emergency kits must be
readily available. This is especially important for outpatient clinics or
offices that continue to sedate patients.
Current AAP/AAPD guidelines
specifically outline recommended discharge criteria to minimize the likelihood
of AEs following sedation. The following six criteria are critical for
achieving safe management of sedated pediatric patients: 1) cardiovascular
function and airways are stable and satisfactory; 2) patient is easily
arousable, with protective reflexes intact; 3) patient can talk (if
appropriate based on age); 4) patient can sit up unaided (if appropriate based
on age); 5) return to normal baseline responsiveness; and 6) hydration status
is adequate.1 Documentation of sedation, the procedure, and
recovery will help to minimize premature discharge. In addition, patients
should be accompanied by at least two adults upon discharge and should have
by-mouth intake evaluated to reduce the risk of aspiration.9 The
administration of sedatives at the home is no longer an acceptable practice,
as it poses too great a risk to the patient.9,20 If patients are
sedated using certain drugs with extended half-lives, they may need longer,
less-intensive observation to ensure that unwanted effects do not occur
several hours after the procedure. The Institute of Medicine has stated that a
national, mandatory reporting system should be in place to help improve
awareness of adverse sedation events in terms of risk factors and causes.
21 The FDA currently administers MedWatch, a nonmandatory vehicle for
collecting data involving AEs. Records of AEs are important to prompt
root-cause analyses.
Management of Adverse
Events
For several years
there has been a great deal of debate as to which drugs should be used in
pediatric sedation, who should be administering doses, and what type of
setting is most appropriate. Is it appropriate to sedate a child for up to two
hours for a radiological procedure that may only last five minutes? The lowest
possible doses of the least number of medications that will produce the
desired effects should be used. By using monotherapy or combinations of drugs
that include reversible agents, we decrease the likelihood that a potentially
harmful adverse event occurring. Drugs such as naloxone (Narcan) for opioid
reversal or flumazenil (Romazicon) for benzodiazepine reversal can be used in
possible overdose or synergistic situations.22 Both drugs can be
titrated fairly quickly to achieve an adequate response: naloxone 0.1 to 0.4
mg IV every three to five minutes and flumazenil 0.2 to 1 mg IV every two to
three minutes. Multiple dose forms (IV, IM [intramuscular], SL [sublingual])
also make these drugs favorable in rescue situations. Providers should use
caution and avoid using these medications if drug dependence is an issue, as
they may instill a rapid onset of withdrawal, which in some cases (with
benzodiazepines, for example) could prove to be fatal. If airway obstruction
is involved, the first step is to determine the cause. If laryngospasm is the
cause and positive pressure using a bag-valve mask has not resolved the issue,
succinylcholine 0.1 to 0.2 mg/kg IV (a neuromuscular blocking agent) should be
added as adjunctive treatment. If anaphylactoid reaction has occurred,
epinephrine 0.3 mg IM should be started. For severe reactions, 0.5 mg may be
needed, and the drug can be titrated in 0.1-mg increments if the patient is
refractory. Albuterol inhalation (2-3 inh q1-2min • 3 if needed
) should be used in conjunction with epinephrine if bronchospasm occurs.
Sedatives will decrease sympathetic outflow from the heart, causing
bradycardia and hypotension. Ephedrine (dilute 10 mg/mL; 5-10 mg IV q5min; max
50 mg) will directly stimulate the release of norepinephrine by acting on both
alpha and beta receptors. It is considered the drug of choice due to its
longer duration of action (60-90 min). Second-line agents atropine (0.5 mg IV,
IM, SL q4-5min • 4-5) and phenylephrine (dilute to 0.1 mg/mL; 0.1 mg IV q3min
• 5) may also be used but generally only produce effects for five to ten
minutes. Caution must also be used with ephedrine and phenylephrine as they
must both be diluted before use. Phenylephrine should be used for hypotension
with tachycardia, as it works selectively as an alpha agonist and will not
increase heart rate while producing venoconstriction and increased pressure.
If hypertension occurs during sedation, nitroglycerin (1 tablet SL q5min • 3),
morphine (2.5 mg IV q3-5min; max 10 mg) or aspirin (325 mg or four 81-mg tabs)
are options. Labetalol (10 mg IV q5min, repeated if needed) can help to
prevent reflex tachycardia but should be avoided in asthmatics due to
nonselectivity. In these situations, esmolol is a better choice.
Discussion
Guidelines endorsed
by several national organizations have helped to reduce the incidence and
likelihood AEs in pediatric sedation; however, events still occur. Malviya et
al found that 28 of 56 reported deaths due to complications related to
sedation occurred after the first publication of AAP guidelines.11
In addition, 27% of the records that were reviewed did not include a sedation
flowsheet, which is required per the guidelines. Strict adherence to published
guidelines is essential to reducing the incidence of sedation-related AEs.
Administration of sedatives should be done by licensed practitioners, assisted
by staff members trained in emergency rescue protocol. New developments in
human simulation are helping to improve sedation techniques, limit events, and
promote safety.23 Accurate knowledge of drugs being used for
sedation can decrease the incidence of adverse events. Pharmacists can play a
vital role in completing medication reconciliation, calculating accurate mg/kg
limits, and selecting appropriate drug therapy. Soft data suggests that drugs
with reversible potential (opioids, benzodiazepines) may have advantages over
other sedatives in reducing the incidence of events.7 Practitioners
should be using the fewest number of drugs at the lowest efficacious dose.
Dexmedetomidine (Precedex), a fairly new medication, may be considered in the
future for sedation monotherapy. The current safety and efficacy profile is
not adequate and further studies are needed to determine its value in moderate
sedation.24 Documentation is the final key step in reducing the
incidence of events. Proper reporting can help institutions as well as larger
consortiums develop root-cause analyses, which can identify potentially
hazardous practices and improve current guidelines.
REFERENCES
1. Maxwell LG, Yaster M. The myth of conscious sedation. Arch Pediatr Adolesc Med. 1996;150:665-667.
2. Pena BM, Krauss B. Adverse events of procedural sedation and analgesia in a pediatric emergency department. Ann Emerg Med. 1999; 34(pt 1):483-491.
3. Law AK, Ng Dk, Chan KK. Use of intramuscular ketamine for endoscopy sedation in children. Pediatr Int. 2003;45:180-185.
4. Malviya S, Voepel-Lewis T, Eldevik OP, et al. Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth. 2000;84:743-748.
5. Pitetti RD, Singh S, Pierce MC. Safe and efficacious use of procedural sedation and analgesia by nonanesthesiologists in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157:1090-1096.
6. Polaner DM, Houch CS, Rockoff MA, et al. Sedation, risk, and safety: do we really have data at last? Pediatrics. 2001;108:1006-1008.
7. Cote CJ, Karl HW, Notterman DA, et al. Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics. 2000;106:633-644.
8. Cravero JP, Blike GT, Beach M, et al. Incidence and nature of adverse events during pediatric sedation/anesthesia for procedures outside the operating room: report from the Pediatric Sedation Research Consortium. Pediatrics. 2006;188:1087-1096.
9. Guideline for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Adopted 2006. Developed and endorsed by the American Academy of Pediatrics and the American Academy of Pediatric Dentistry. 115-132.
10. Cote CJ, Wilson S. Guidelines for the monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatrics. 2006;118:2587-2602.
11. Malviya S, Voepel-Lewis T, Tait AR. Adverse events and risk factors associated with the sedation of children by nonanesthesiologists. Anesth Analg. 1997;85:1207-1213.
12. Cote CJ, Daniel A, Notterman HW, et al. Adverse sedation events in pediatrics: a critical incident analysis of contributing factors. Pediatrics. 2000;105:805-814.
13. DRUGDEX Healthcare Series [internet database]. Greenwood Village, CO: Thomson Healthcare. Updated periodically.
14. Guideline on appropriate use of nitrous oxide for pediatric dental patients. Adopted 2005. Developed and endorsed by the American Academy of Pediatric Dentistry.
15. Moore PA. Adverse drug reactions in dental practice: interactions associated with local anesthetics, sedatives, and anxiolytics. J Am Dent Assoc. 1999;130:541-544.
16. Bates DW, Teich JM, Lee J, et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc. 1999;6:313-321.
17. Mayers DJ, Hindmarsh KW, Sankaran K, et al. Chloral hydrate disposition following single-dose administration to critically ill neonates and children. Dev Pharm Ther. 1991;16:71-77.
18. Runciman WB, Webb Rk, Barker L, Currie M. The Australian Incident Monitoring Study. The pulse oximeter: applications and limitations--an analysis of 2000 incident reports. Anaesth Intensive Care.1993;21:543-550.
19. Cravero JP, Blike GT. Review of pediatric sedation. Anesth Analg. 2004;99:1355-1364.
20. Guideline on use of anesthesia personnel in the administration of office-based deep sedation/general anesthesia to the pediatric dental patient. Adopted 2001. Revised 2005, 2007. Developed and endorsed by the American Academy of Pediatric Dentistry.
21. Richardson WC, Berwick DM, and the Committee on Quality of Health Care in America. To err is human: building a safer health system. Washington, DC: National Academies Press; 2000.
22. Becker DE, Haas, DA. Management of complications during moderate and deep sedation: respiratory and cardiovascular considerations. Anesth Prog. 2007;54:59-69.
23. Farnsworth ST, Egan SE, Westenskow D. Teaching sedation and analgesia with simulation. J Clin Monit Comput. 2000;16:273-285.
24. Phan H, Nahata MC. Clinical use of dexmedetomidine in pediatric patients. Paediatr Drugs. 2008;10:49-69.
25. Taketomo CK, Hodding JH, Kraus
DM. Pediatric Dosage Handbook. Lexi-Comp; 2006.
To comment on this article, contact
editor@uspharmacist.com.





