US
Pharm. 2006;6:52-60.
Clostridium difficile
is an anaerobic, gram-positive, spore- and toxin-producing bacillus that was
first discovered in 1935. At that time, it was considered to be normal flora
in neonates, and the organism¡¯s role in antibiotic-associated diarrhea was not
elucidated until the 1970s.1 Since then, C. difficile
¨Cassociated diarrhea (CDAD) has become one of the most common and problematic
nosocomial infections to contain. Recently, the pathophysiology and
epidemiology of CDAD have been changing. Noteworthy developments include
reports of more severe CDAD, cases occurring in settings not associated with
health care, and a potential reduction in treatment effectiveness. This
article will provide a brief review of traditional CDAD, as well as summarize
the recent noteworthy changes in disease that pharmacists should recognize.
Background
The classic
intestinal manifestations of CDAD consist of three or more episodes of watery
diarrhea per day, fever, leukocytosis, and abdominal pain or cramping.2
While diarrhea is the most common manifestation, CDAD symptoms can range all
the way from asymptomatic carriage to severe disease characterized by
pseudomembranous colitis, toxic megacolon, colonic perforation, and death. In
pseudomembranous colitis, the colonic mucosa is studded with raised white and
yellowish plaques that may coalesce to form pseudomembranes (composed of
mucin, neutrophils, fibrin, and cellular debris) that can exist throughout the
entire colon. Toxin-secreting C. difficile is associated with 10% to
30% of cases of antibiotic-associated diarrhea, 50% to 70% of cases of
antibiotic-associated colitis, and greater than 90% of cases of
antibiotic-associated pseudomembranous colitis.2-4
The development of CDAD is a
multistep process that involves alteration of normal colonic flora by
antibacterial agents, colonization by and overgrowth of toxin-secreting
isolates of C. difficile, and subsequent expression of toxin, which
leads to mucosal injury and inflammation. Normal gut flora confers protection
against infection with C. difficile, as animal models suggest that
normally present gut bacteria limit carbon sources required for C. difficile
overgrowth.4 Prior antibacterial use traditionally predisposes a
person to development of CDAD, as it disrupts the normal gut flora. In most
cases, alteration of normal flora precedes colonization of the colon via the
fecal¨Coral route. The acid-resistant C. difficile spores enable
the organism to pass through the acidic medium of the stomach unharmed. In the
duodenum, they are exposed to primary bile acid necessary for the conversion
to vegetative forms. It should be noted that alteration of normal colonic
flora does not always precede colonization with C. difficile via the
fecal¨Coral route. Many pediatric and adult patients have been identified as
asymptomatic carriers. When CDAD develops in such cases, it is often due to
overgrowth of resistant C. difficile subsequent to selection by
antibacterial use. As C. difficile multiplies, it produces two toxins
(A and B) that cause diarrhea and colitis. The two toxins are internalized by
intestinal epithelium and ultimately cause cytoskeletal disruption, cell
rounding and retraction, opening of tight junctions of intestinal epithelial
cells, and apoptosis. Toxins A and B also induce inflammation and secretory
responses via various inflammatory mediators and neutrophil chemotaxis.3,4
Most cases of CDAD have been
associated with nosocomial transmission. Surveillance studies have shown that
20% to 30% of hospitalized patients typically become colonized with C.
difficile, with approximately one third proceeding to develop CDAD.2,4
Historically, there have been reports of CDAD being associated with
community-acquired diarrhea as well; however, in most cases these patients had
recent previous exposure to health care resources (e.g., hospitalization or
nursing home).5 Health care worker hand carriage with transfer from
patient to patient likely accounts for the majority of nosocomial
transmission. Contact with contaminated fomites (e.g., telephones, bedpans,
rectal thermometers) has been implicated in the spread of C. difficile
as well. Further, despite environmental cleaning, the persistence of spores
for weeks to months after a patient has left the environment also contributes
to horizontal transmission.5
Classic risk factors for
developing CDAD include hospitalization, advanced age, chronic comorbidity,
gastrointestinal surgery or manipulations, tube feedings, use of anti-acid
drugs (particularly proton pump inhibitors), and preeminently, previous
exposure to antimicrobial agents (including antineoplastic agents with
antimicrobial activity).5-11 While virtually all antimicrobials
have been associated with development of CDAD, agents such as clindamycin,
aminopenicillins, and cephalosporins historically have been considered to pose
the greatest risk.4,5,8,12 This strong association of CDAD with the
use of certain antibiotics is due to selection of resistant C. difficile
. In many epidemics of CDAD, the circulating clone was found to be resistant
to antibiotics that were considered to be risk factors for the outbreak, such
as clindamycin.12 Subsequent reduction in the use of the antibiotic
identified as selecting resistant C. difficile resulted in a decrease
in CDAD.13
Pharmacotherapy of CDAD
Treatment of CDAD
generally includes the administration of oral metronidazole or vancomycin. If
possible, the offending antibiotic should be discontinued. In severe disease,
colonoscopy and surgical intervention may be necessary. Studies conducted
during the late 1970s and 1980s found similar clinical cure rates between
metronidazole and vancomycin, ranging from 86% to 98%.14 Due to
concerns about the potential for oral vancomycin to foster the development of
vancomycin-resistant Enterococcus and the equivalence of the two agents in
clinical trials, national guidelines published during the 1990s recommended
oral metronidazole as the agent of choice and vancomycin use only in cases of
metronidazole failure or severe or life-threatening disease.5 The
recommended dosing for metronidazole is 250 mg QID or 500 mg TID for 10 days.
Oral vancomycin is typically dosed at 125 mg QID for 10 days. Metronidazole
and vancomycin regimens are generally well tolerated.2 The most
common adverse effects of metronidazole therapy include a disulfiram-like
reaction, if alcohol is used concurrently, and loss of appetite or taste
disturbance.
Changing Prevalence of CDAD
The prevalence rate
of CDAD has changed notably since the 1970s. An analysis of hospital data from
the National Nosocomial Infectious Surveillance System identified an upward
trend in rates of CDAD from the mid to late 1980s to 2001.15 More
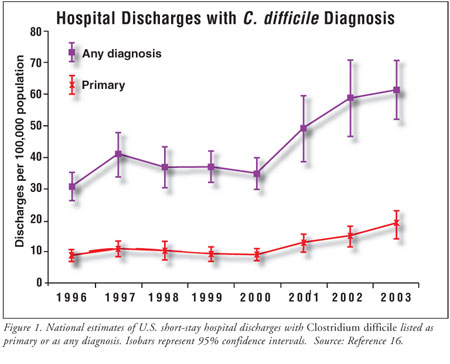
recently, CDAD rates have increased with greater intensity. From 2000 to 2001,
rates of CDAD on U.S. hospital discharge diagnoses increased by 26% (figure 1).
16 One locale, the University of Pittsburgh Medical Center¨CPresbyterian,
reported that the incidence of CDAD in 2000 and 2001 was nearly double the
incidence from 1990 to 1999.17 In Quebec, rates almost quadrupled,
from 35.6 to 156.3 per 100,000 population.18
Historically, the prevalence of CDAD in outpatient populations has been low,
with the overall risk assessed at less than one case per 10,000 outpatient
prescriptions for antibiotics.19 In May and June of 2005, 23 cases
of community-acquired CDAD (CA-CDAD) were reported from Pennsylvania, Ohio,
New Hampshire, and New Jersey.19 The cases occurred in patients
thought to be at low risk (no or low exposure to a health care setting) for
CDAD. The estimated annual incidence in Philadelphia and surrounding areas was
7.6 cases per 100,000 population, and one case per 5,549 antibiotic
prescriptions. The increase in prevalence rates of CDAD in both the nosocomial
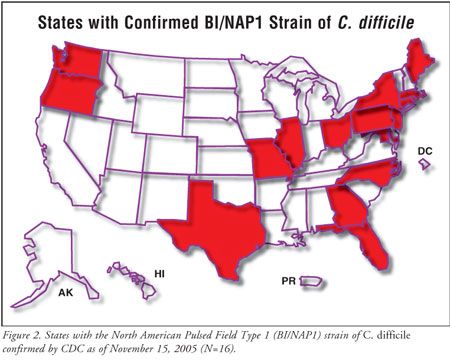
and community setting appear to correspond with the widespread emergence of a
previously uncommon strain of C. difficile termed BI/NAP1 (figure 2).
20
The Emergence of the C. difficile BI/NAP1 Strain
McDonald et al. recently published
findings on 187 C. difficile isolates collected from health care
facilities with CDAD outbreaks in eight states between 2000 and 2003.21
Molecular analysis was used to compare these strains to more than 6,000
isolates collected prior to 2001. The BI/NAP1 strain was determined to
constitute one half of the 2000¨C2003 isolates, whereas this strain was
identified in only 17% of the historical isolate collection. The BI/NAP1
strain contains a different toxin called binary toxin. While the specific role
of binary toxin as a virulence factor is unknown and under investigation,
isolates that contain the gene for binary toxin also frequently were missing a
gene (tcdC) that serves as a negative regulator for production of classic
C. difficile toxins A and B. BI/NAP1 isolates have been shown to produce
16 and 23 times more A and B toxins in vitro, respectively, than have previous
epidemic strains.22 It is important to note that while binary toxin
itself has not been established as a virulence factor, researchers noted that
the prevalence of the binary toxin was higher in isolates from outbreaks
associated with increased morbidity, including those hospitals reported by
McDonald.21
Changing Severity of Disease
There are now
numerous reports of both increasing severity of CDAD and increased
attributable mortality since 2001.17-19,21,23,24 In 2002 in Quebec,
complicated cases (defined as death within 30 days, megacolon, perforation,
colectomy, or shock requiring vasopressors) increased fourfold over rates
among historical controls dating back to 1989.23 Thirty-day
mortality also increased from 4.7% to 13.8% during the same study period. In
2000¨C2001, the epidemic at University of Pittsburgh Medical
Center¨CPresbyterian reported a doubling in life-threatening CDAD infections,
identifying 64 patients who required colectomies or died of CDAD.17,25
Typically, historical reports of attributable mortality due to CDAD have been
<3%. However, several studies have recently reported attributable mortality as
high as 16.7% and overall mortality as high as 37%.17,18,23,24 The
CA-CDAD cases described above also had a 30% overall hospitalization rate and
included reports of severe CDAD, including death.19
Changing Antibiotic Risk
Factors Associated with CDAD
Paralleling the
emergence of the BI/NAP1 C. difficile strain, fluoroquinolones are
increasingly being implicated as risk factors for development of CDAD.
25-28 In some cases, older fluoroquinolones such as ciprofloxacin and
levofloxacin have been implicated; however, several reports of association of
CDAD with gatifloxacin and moxifloxacin, which possess antianaerobic activity,
are concerning.26,28 Analysis of antimicrobial spectra may
partially explain this association as both moxifloxacin and gatifloxacin
possess greater anaerobic activity than levofloxacin and ciprofloxacin, thus
incurring greater disruption of colonic flora and a subsequent increase in the
likelihood of developing CDAD. Gaynes et al., analyzing a CDAD outbreak in a
Veterans Affairs (VA) long-term-care facility, reported a sharp increase in
CDAD after a formulary conversion from levofloxacin to gatifloxacin.26
Use of gatifloxacin, as well as of clindamycin, was highly correlated with
CDAD. The outbreak terminated upon a formulary change back to levofloxacin.
The previously reported outbreaks in Quebec and in Pittsburgh also reported
that certain fluoroquinolones, as well as select cephalosporins and
clindamycin, were associated with CDAD.25,28 In the Quebec
outbreak, Pepin and colleagues reported that patients given gatifloxacin
versus other fluoroquinolones were at greater risk for CDAD.28
Further, fluoroquinolone administration for as short as one to three days was
associated with CDAD, whereas most other antibiotics associated with increased
risk of CDAD required more than three days. Finally, the aforementioned
McDonald study reported that resistance to gatifloxacin and moxifloxacin was
virtually 100% in BI/NAP1 isolates compared to 42% of non¨CBI/NAP1 isolates.
21 Seventy-nine percent of both groups of isolates were resistant to
clindamycin. In summary, fluoroquinolones have emerged as strong risk factors
for the development of CDAD, especially disease caused by the BI/NAP1 strains,
and newer fluoroquinolones with antianaerobic activity may pose the greatest
risk.
Potentially Decreased
Effectiveness of CDAD Treatment
Treatment of CDAD
has not changed appreciably in the past 25 years. In the early 1980s, oral
vancomycin was considered the standard of care, with a response rate
approximating 80% to 90%. Metronidazole was also shown to be comparably
efficacious. Due to concerns about oral vancomycin use increasing risk for
vancomycin-resistant Enterococcus spread, metronidazole became the agent of
choice for most cases of CDAD.5 In the past five years, concern has
surfaced over the efficacy of the current treatment modalities. The
aforementioned study from Quebec posted alarming treatment failure rates,
suggesting problems with efficacy of metronidazole: Use of vancomycin as a
¡°rescue¡± therapy remained at 9.6% from 1991 to 2002 but increased by more than
twofold to 25.7% in 2003 to 2004.23 In addition, the recurrence
rate of patients treated with metronidazole increased from 20.8% to 47.2%
during the same period. Further analysis tempers the reports of alarming
recurrence rates. Researchers discovered that of the 463 patients who
experienced a first recurrence of CDAD, most subsequent infections were
reinfections rather than relapses.29 In addition, neither
metronidazole nor vancomycin was associated with a higher recurrence rate or
more severe initial disease. However, in patients with advanced age, WBC ¡Ý
20,000 and serum creatinine ¡Ý2.2 mg/dL were associated with complicated
CDAD, irrespective of treatment. Another study from a Texas VA hospital also
suggested the efficacy of metronidazole to be approximately 50%, markedly
different from the 90% reported in the 1980s.30
Reasons for the apparent lack
of efficacy of current treatment modalities are not clear. Few in vitro
studies have suggested that the minimum inhibitory concentration of
metronidazole for C. difficile is higher in some strains; however,
susceptibility data for metronidazole and vancomycin were not reported for the
Quebec and Texas VA studies.14 In addition, no evidence exists
indicating that metronidazole resistance plays a clinically important role in
treatment failures and recurrence. Gerding suggested that the variability in
clinical failure rates in the aforementioned studies may be related to the
observational nature and differences in case definitions and clinical end
points.31 In addition, the highly virulent strains identified may
have factors that predispose infected individuals to relapses of CDAD.
Finally, the highly virulent strains of C. difficile may lengthen
hospitalization and thus increase the risk of becoming reinfected and
redeveloping CDAD.29 In summary, while there are several recent
reports of increasing clinical failures and relapses, it is unclear if these
findings represent loss of antibiotic potency, increased disease virulence, or
differences in clinical reporting methods between trials. However, the
clinician should be aware of the possibility of increased CDAD treatment
failures with standard therapies.
Potential Impact on
Diagnosis, Prevention, and Treatment
The emergence of
increasingly severe CDAD has several implications for its diagnosis and
treatment. First, for patients (both inpatients and outpatients) who develop
diarrhea and have any risk factors for CDAD, early diagnosis and treatment are
critical to prevent morbidity and mortality. Pharmacists in both hospital and
community settings are capable of contributing to early diagnosis and
treatment, as they are aware of risk factors (especially antibiotic risk
factors). In a community setting, pharmacists often receive requests from
patients who seek nonprescription products for relief of diarrhea. Pharmacists
should inquire about recent antibiotic use by the patient or the immediate
family members (siblings, etc.) as part of the patient assessment and should
consider referral for further medical evaluation if CDAD risk and symptoms are
present. Patients with CDAD should not receive antimotility antidiarrheal
agents, such as loperamide or diphenoxylate, as these may worsen CDAD.2
Prevention and control of CDAD
are vital in all patient care settings. All patients being treated for CDAD
and their caregivers should be reminded to wash their hands regularly with
soap and warm water.32 It is important to note that the use of
alcohol-based disinfecting hand rub solutions are not recommended, as the
solutions are not reliably sporicidal and will not prevent spread of C.
difficile spores as well as traditional hand washing. Cleaning and
disinfection of environmental surfaces (e.g., bedpans, light switches,
bathtubs) are also recommended, since C. difficile spores may last for
weeks to months. Various disinfecting agents, such as unbuffered hypochlorite
or formaldehyde and glutaraldehyde, have been employed, and further study is
merited to determine which disinfectants are most effective. While
environmental disinfection practices are standard in institutional settings, a
10% bleach solution is affordable, is readily prepared at home, and is an
effective disinfectant for household use. Given the aggressive nature of
BI/NAP1 C. difficile strains and the recent reports of CA-CDAD, it is prudent
to recommend these practices to outpatients being treated for CDAD and to
their caregivers.
Since asymptomatic carriers
harbor C. difficile, some clinicians have attempted to eliminate
asymptomatic carriage by administration of metronidazole or vancomycin. Due to
metronidazole¡¯s virtually complete absorption by those without diarrhea, it is
ineffective in eliminating asymptomatic carriage, as intraluminal
concentrations are negligible.33 Further, while oral vancomycin
eliminates carriage effectively (due to high fecal concentrations even during
normal gut motility), recolonization with C. difficile is common in
patients. Given these findings, use of metronidazole or vancomycin for
prevention by eliminating asymptomatic carriage is not recommended.5
Antimicrobial therapy has been
identified as the preeminent risk factor for the development of CDAD, and
restriction of certain antibiotics has been shown to interrupt epidemics.
Various studies at hospitals throughout the U.S. have shown that restriction
of clindamycin decreased the incidence of CDAD associated with
clindamycin-resistant epidemic strains.13 The emergence of CDAD
caused by fluoroquinolones may necessitate similar restriction of
fluoroquinolone use, at least during CDAD epidemics.26 Use of
antimicrobials that possess lower risk for developing CDAD than do
gatifloxacin and moxifloxacin (cotrimoxazole, tetracyclines, macrolides, etc.)
may be preferable for the treatment of many uncomplicated infections,
particularly in outpatients without recent antibiotic exposure. Pharmacists
are encouraged to discuss these options for treatment with patients and health
care providers, when appropriate.
The most recent reports from Quebec
tend to support the current Standard of Care, which suggests that initial or
first recurrences of mild to moderate CDAD be treated with metronidazole, with
vancomycin reserved for severe or refractory disease.5,29 In such
cases, use of oral vancomycin seems prudent if the patient¡¯s condition
continues to deteriorate or WBC counts rise after metronidazole is started.
31 In addition, increased monitoring of metronidazole efficacy in such
cases may be necessary to detect treatment failure or sequelae of C.
difficile infection. Further, development of ileus or fulminant CDAD may
require surgery, in addition to more aggressive treatment such as intravenous
metronidazole coupled with vancomycin enemas.13 Recurrent CDAD is a
problem for which no clear consensus has emerged. Repeating treatment courses
with high-dose vancomycin has proven efficacious, while others employ pulsed
dosing, believing that C. difficile spores will germinate between
pulses and be susceptible to the next dose of drug.34,35
Conclusion
In the past five
years, the pathophysiology and epidemiology of CDAD have changed. Noteworthy
developments include reports of more severe CDAD, cases occurring in settings
not associated with health care, and a potential reduction in treatment
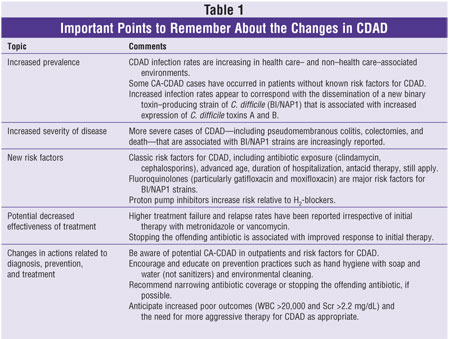
effectiveness. table 1 highlights the take-home points to remember about the
recent findings involving CDAD. The changing nature of CDAD presents new
challenges for patient care in which effective drug therapy is necessary, and
a number of new antibiotic as well as nonantibiotic therapies are in
development. As professionals versed in drug therapy, pharmacists are
positioned to contribute to effective patient care by evaluating patient risk
for CDAD and providing recommendations regarding prevention and effective drug
therapy for all severities of CDAD.
References
1. Tedesco FJ, Barton
RW, Alpers DH. Clindamycin-associated colitis: a prospective study. Ann
Intern Med. 1974;81:429-433.
2. Thielman NM, Wilson
KH. Antibiotic-associated colitis. In: Mandell, Douglas, and Bennett¡¯s
Principles and Practice of Infectious Diseases. Philadelphia: Elsevier
Churchill Livingstone; 2005:1249-1259.
3. Mylonakis E, Ryan
ET, Calderwood SB. Clostridium difficile¨Cassociated diarrhea: a review.
Arch Intern Med. 2001;161:525-533.
4. Bartlett JG.
Clinical practice: antibiotic-associated diarrhea. N Engl J Med.
1996;346:334-339.
5. Gerding DN, Johnson
S, Peterson L, et al. Clostridium difficile¨Cassociated diarrhea and colitis.
SHEA Position Paper. Infect Control Hosp Epidemiol. 1995;16:459-477.
6. Manabe YC, Vinetz
JM, Moore RD, et al. Clostridium difficile colitis: an efficient approach to
diagnosis. Ann Intern Med. 1995;123:835-840.
7. Bliss DZ, Johnson S,
Savik K, et al. Acquisition of Clostridium difficile and Clostridium
difficile-associated diarrhea in hospitalized patients receiving tube feeding.
Ann Intern Med. 1998;129:1012-1019.
8. Oldfield EC 3rd.
Clostridium difficile-associated diarrhea: risk factors, diagnostic methods,
and treatment. Rev Gastroenterol Disord. 2004;4:186-195.
9. Kyne L, Sougioultzis
S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor
for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol
. 2002;23:653-659.
10. Dial S, Alrasadi K,
Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital
inpatients prescribed proton pump inhibitors: cohort and case-control studies.
CMAJ. 2004;171:33-38.
11. Dial S, Delaney JA,
Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of
community-acquired Clostridium difficile-associated disease. JAMA.
2005;294:2989-2995.
12. Johnson S, Samore
MH, Farrow KA, et al. Epidemics of diarrhea caused by a clindamycin-resistant
strain of Clostridium difficile in four hospitals. N Engl J Med.
1999;341:1645-1651.
13. Davey P, Brown E,
Fenelon L, et al. Interventions to improve antibiotic prescribing practices
for hospital inpatients. Cochrane Database Syst Rev. 2005;(4):CD003543.
14. Aslam S, Hamill RJ,
Musher DM. Treatment of Clostridium difficile-associated disease: old
therapies and new strategies. Lancet Infect Dis. 2005;5:549-557.
15. Archibald LK,
Banerjee SN, Jarvis WR. Secular trends in hospital-acquired Clostridium
difficile disease in the United States, 1987¨C2001. J Infect Dis.
2004;189:1585-1589.
16. McDonald CL, Owings
M, Jernigan DB. Clostridium difficile infection in patients discharged from US
short-stay hospitals, 1996¨C2003. Emerg Infect Dis. 2006;12(3).
Available from www.cdc.gov/ncidod/EID/vol12no03/05-1064.htm.
17. Dallal RM,
Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an
underappreciated and increasing cause of death and complications. Ann Surg
. 2002;235:363-372.
18. Pepin J, Valiquette
L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of
Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ.
2004;171:466-472.
19. Centers for Disease
Control and Prevention (CDC). Severe Clostridium difficile-associated disease
in populations previously at low risk¡ªfour states, 2005. MMWR Morb
Mortal Wkly Rep. 2005;54:1201-1205.
20. Centers for Disease
Control and Prevention. Clostridium difficile Infections. Available at:
www.cdc.gov/ncidod/dhqp/id_Cdiff_data.html. Accessed February 15, 2006.
21. McDonald LC,
Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of
Clostridium difficile. N Engl J Med. 2005;353:2433-2441.
22. Bartlett JG, Perl
TM. The new Clostridium difficile¡ªwhat does it mean? N Engl J Med
. 2005;353:2503-2505.
23. Pepin J, Alary ME,
Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium
difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591-1597.
24. Pepin J, Valiquette
L, Cossette B. Mortality attributable to nosocomial Clostridium
difficile-associated disease during an epidemic caused by a hypervirulent
strain in Quebec. CMAJ. 2005;173:1037-1042.
25. Muto CA, Pokrywka
M, Shutt K, et al. A large outbreak of Clostridium difficile-associated
disease with an unexpected proportion of deaths and colectomies at a teaching
hospital following increased fluoroquinolone use. Infect Control Hosp
Epidemiol. 2005;26:273-280.
26. Gaynes R, Rimland
D, Killum E, et al. Outbreak of Clostridium difficile infection in a long-term
care facility: association with gatifloxacin use. Clin Infect Dis.
2004;38:640-645.
27. McCusker ME, Harris
AD, Perencevich E, Roghmann MC. Fluoroquinolone use and Clostridium
difficile-associated diarrhea. Emerg Infect Dis. 2003;9:730-733.
28. Pepin J, Saheb N,
Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk
factor for Clostridium difficile-associated diarrhea: a cohort study during an
epidemic in Quebec. Clin Infect Dis. 2005;41:1254-1260.
29. Pepin J, Routhier
S, Gagnon S, Brazeau I. Management and outcomes of a first recurrence of
Clostridium difficile-associated disease in Quebec, Canada. Clin Infect Dis
. 2006;42:758-764.
30. Musher DM, Aslam S,
Logan N, et al. Relatively poor outcome after treatment of Clostridium
difficile colitis with metronidazole. Clin Infect Dis.
2005;40:1586-1525.
31. Gerding DN.
Metronidazole for Clostridium difficile-associated disease: is it okay for
Mom? Clin Infect Dis. 2005;40:1598-1600.
32. Boyce JM, Pittet,
D. Guideline for hand hygiene in health-care settings. MMWR Recomm Rep.
2002;51(RR16):1-45.
33. Johnson S, Homann
SR, Betin KM, et al. Treatment of asymptomatic Clostridium difficile carriers
(fecal excretors) with vancomycin or metronidazole. Ann Intern Med.
1992;117:297-302.
34. Tedesco FJ, Gordon
D, Fortson WC. Approach to patients with multiple relapses of
antibiotic-associated pseudomembranous colitis. Am J Gastroenterol.
1985;80:867-868.
35. McFarland LV, Elmer
GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of
recurrent Clostridium difficile disease. Am J Gastroenterol.
2002;97:1769-1775.
To comment on this article,
contact
editor@uspharmacist.com.






