US Pharm. 2014;39(7)(Specialty&Oncology suppl):8-11.
ABSTRACT: Peripheral arterial disease (PAD) is a common condition defined as arterial insufficiency of the peripheral circulation. Impairment in circulation can result in symptoms, functional impairment, and decreased survival, but in many cases will remain asymptomatic. The hallmark feature of PAD is intermittent claudication, a condition wherein pain is exacerbated and consistently reproducible upon physical exertion. PAD is initially managed by supervised exercise programs, risk factor reduction, and medications for those with symptoms of intermittent claudication. Surgical intervention may be necessary for those with severe symptoms not responsive to initial therapy.
Peripheral arterial disease (PAD) is defined as arterial insufficiency of the peripheral circulation that can affect any blood vessels distal to the aorta. Most commonly, this includes the femoropopliteal, tibial, carotid, vertebral, splenic, renal, and brachiocephalic arteries. PAD is generally a sign of widespread atherosclerosis and is therefore closely linked to coronary artery disease. As occlusion and obstruction worsens, a patient diagnosed with PAD has an increased risk for cardiac complications in addition to complications in the extremity where the disease is detected.1
Epidemiology
PAD likely affects a large portion of the U.S. population. It is difficult to estimate prevalence, however, as those who are asymptomatic often go undiagnosed. Symptomatic disease in the form of intermittent claudication is estimated to have a prevalence of 4.5%, while asymptomatic patients may be as prevalent as 25%. 2,3 Rates of critical limb ischemia, a severe complication of PAD, are estimated to be about 1%.4
Pathophysiology
While there are many possible causes for arterial insufficiency, PAD is the most common. The pathophysiology for atherosclerosis in PAD is essentially the same as in coronary artery disease, although it occurs in different vessels. Lipoproteins first enter the sub-endothelial space of the vasculature, where they are oxidized. This causes endothelial dysfunction, which leads to macrophage invasion of the vessel wall. Macrophages accumulate as foam cells, which form fatty streaks and lead to a disruption in factors such as nitric oxide and various growth factors. This disruption leads to cellular proliferation, migration into the intima of the vessel, and plaque development.5
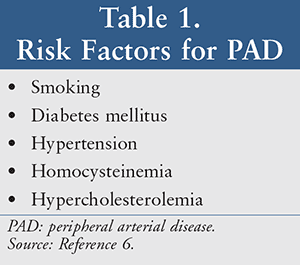
Atherosclerosis will be asymptomatic in PAD, as it is in coronary artery disease, until the oxygen demands of the target muscles cannot be met due to the arterial occlusion. As this occurs, the development of claudication symptoms or critical limb ischemia can eventually occur. Risk factors for PAD are the same as those for coronary artery disease (TABLE 1). 6
Clinical Presentation
Intermittent claudication is the hallmark of patients with symptomatic PAD. It is characterized by recurrent, reproducible pain in the lower extremities that is exacerbated by exercise and relieved by rest. The amount of effort that exacerbates the distress is generally consistent and is located in the muscle distal to the obstructed artery. Patients may describe their symptoms as cramping, numbness, weakness, or aching. Upon rest, the symptoms usually resolve predictably within 5 minutes.6
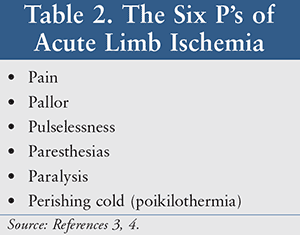
Critical limb ischemia is a complication that occurs with worsening severity of PAD. Patients with critical limb ischemia may experience pain at rest, tissue loss, and possibly ulceration. Signs of acute limb ischemia are listed in TABLE 2. Swift action must be taken if acute limb ischemia is present because the viability of the limb is in jeopardy.3 ,4
Screening and Diagnosis
Screening for PAD can include functional assessment, hemodynamic monitoring, and anatomical screening. Functional assessment such as walking on a treadmill to exacerbate claudication is important to identify the degree of impairment caused by a stenotic plaque. One of the most popular ways to obtain an objective measure of functional exercise capacity is the 6-minute walk test. The test is easy to administer, as it involves no equipment besides a 100-foot straight hallway with a hard, flat floor. The patient is instructed to walk as far as he or she can in 6 minutes. This enables practitioners to measure the effects of nonpharmacologic or pharmacologic interventions in PAD over time. Additionally, the 6-minute walk test is demonstrated to be a predictor of mortality in other disease states.6 ,7
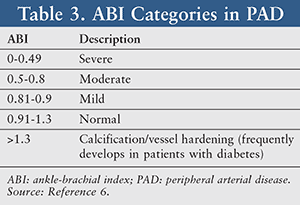
Hemodynamic monitoring assesses the gradient of pressures across a stenotic lesion. This can be done in a noninvasive manner by placing pneumatic cuffs and a probe over the skin at various locations (such as the arms, thighs, calves, ankles, and possibly toes). The pressure at each location can be divided by a reference pressure, creating a ratio. The most commonly used ratio is the ankle-brachial index (ABI), which is obtained by dividing the blood pressure in the ankle by the blood pressure in the brachial artery. A normal ABI is generally considered 0.91 to 1.3, and lower ABIs are highly sensitive and specific for PAD. As PAD worsens, the ABI will decrease as described in TABLE 3.6
Anatomical screening includes angiography, magnetic resonance angiography, and ultrasonography, which can be invasive and may require the use of radioactive dye.6 Since diagnosis is generally made by a history of symptoms of PAD in combination with physical examination findings, vascular imaging is usually not necessary. Anatomical screening may be useful when it is necessary to rule out other etiologies for a patient’s symptoms, such as thromboembolism, arterial aneurysm, and arterial dissection.
Recommendations for screening for PAD with the ABI vary by organization even though it is an inexpensive and noninvasive test and has a high sensitivity and specificity. The U.S. Preventive Services Task Force does not recommended universal screening of asymptomatic patients due to insufficient evidence.8 The American College of Cardiology Foundation and American Heart Association (ACCF/AHA) joint guideline recommends the ABI for detecting PAD in patients at high risk or with symptoms suggestive of PAD. It also states that this is a reasonable tool for patients at intermediate risk to determine cardiovascular risk assessment.9
Management
Treatment for PAD focuses on reducing symptoms, preventing further progression of the disease, and reducing overall cardiovascular risk. In most cases, lifestyle changes, exercise, and claudication medications are enough to slow the progression and improve the symptoms of PAD.
Nonpharmacologic Interventions
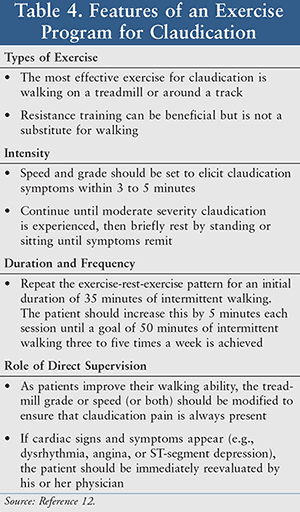
Exercise Programs: Patients with PAD have diminished functional capacity due to the leg symptoms they experience, which prevent them from walking long distances.10 This functional impairment leads to progressive disability and increased cardiovascular risk. Exercise training can benefit patients with PAD by increasing effective blood flow to the legs and increasing the delivery of oxygen.11 The ACCF/AHA recommends supervised exercise programs as initial treatment for lifestyle-limiting symptoms related to PAD.11 The key elements of a therapeutic exercise training program are shown in TABLE 4.12
In a 2008 Cochrane review, the effects of exercise programs were studied with regard to the reduction of symptoms on walking and the improvement in quality of life.11 Any exercise programs for the treatment of intermittent claudication were analyzed, including walking, skipping, and running. Exercise was shown to have a significant benefit, with improvements in maximal walking time, walking ability, and walking distance. These improvements were sustained for up to 2 years.11
Recent evidence demonstrates benefits of exercise training even among those patients with PAD who do not have claudication. McDermott et al conducted a randomized trial of supervised treadmill exercise compared to strength training and usual care in patients with PAD.13 Patients had either claudication or atypical symptoms or were asymptomatic. At 6 months, the treadmill group demonstrated an ability to walk a longer 6-minute distance (+20.9 m) compared to a decline in the control group (-15 m). Lower-extremity resistance training improved leg strength as well as maximum treadmill walking time; however, an increase in 6-minute walk distance was not demonstrated. Both treadmill and resistance exercise training improved physical functioning–associated quality-of-life measures.10 ,12
Smoking Cessation: Patients who smoke and use tobacco products can have up to four times the risk of developing PAD than nonsmokers.14 All patients should be asked about tobacco use at every visit. Smoking cessation interventions and counseling should be offered for those who smoke or use tobacco products. Patients should be assisted through counseling to develop a plan for quitting that includes both behavioral and pharmacologic treatment options for those without contraindications.9 Observed clinical data indicate that smoking cessation is associated with overall increased walking time and improved cardiovascular outcomes.4 ,14
Pharmacologic Intervention
Antiplatelet Therapy: For patients with lifestyle-limiting intermittent claudication refractory to exercise therapy and smoking cessation, the addition of cilostazol (100 mg twice daily) is recommended by the American College of Chest Physicians (ACCP) .15 Cilostazol is a phosphodiesterase II inhibitor that suppresses platelet activation and relaxes vascular smooth muscle. Although it has not been shown to improve overall mortality, it is inferred to improve overall physical function, as an improved score was demonstrated on the physical health subscale of the Short Form Health Survey compared to placebo.15 It has also been shown to improve intermittent claudication symptoms and increase maximal pain-free walking distances in patients by at least 50% compared to placebo or pentoxifylline.4 Cilostazol is contraindicated in patients with a history of heart failure.16 Although it is an antiplatelet agent, it can be added to therapy in patients already on aspirin or clopidogrel, as studies of bleeding risk that compared triple therapy with aspirin, clopidogrel, and cilostazol with aspirin and clopidogrel alone failed to demonstrate or exclude an effect on major bleeding risk.15,16
Pentoxifylline , another antiplatelet agent, has been shown to be less effective then cilostazol when the two agents were studied head-to-head for the treatment of intermittent claudication.4 Mean maximal walking distance of cilostazol-treated patients was significantly greater compared with that of patients who received pentoxifylline or placebo. Pentoxifylline failed to demonstrate a difference in quality of life and was associated with more adverse events, mainly nausea, when compared to placebo.15 ,17 Pentoxifylline should be considered a second-line alternative, after cilostazol for treatment of intermittent claudication.
Although neither aspirin nor clopidogrel has been shown to improve symptoms, antiplatelet therapy is recommended to reduce the risk of myocardial infarction (MI), stroke, and other vascular death in patients with both symptomatic and asymptomatic PAD. The ACCP Evidence Based Practice Guidelines for Antithrombotic Therapy in Peripheral Artery Disease recommend aspirin (75-100 mg/day) for primary prevention in patients aged >50 years with asymptomatic PAD.15 Aspirin was shown to slightly reduce total mortality when taken over 10 years, regardless of cardiovascular risk profile. In moderate-to-high–risk cardiovascular patients, the reduction in MI is closely balanced with a risk of significant bleeding. In this population, the risk and benefit must be carefully weighed.
For secondary prevention in patients with symptomatic PAD, the recommendation is long-term aspirin therapy (75-100 mg/day) or clopidogrel (75 mg/day) .15 A meta-analysis of 16 randomized trials showed that in this high-risk population, aspirin significantly reduced total mortality, nonfatal MI, and nonfatal stroke, with an increased number of nonfatal extracranial bleeds. However, the number of vascular events and deaths prevented outweighed the number of bleeding events with aspirin.18
Clopidogrel can be considered if there is a contraindication to aspirin therapy or a patient has experienced a cardiovascular event while receiving aspirin therapy.4 Clopidogrel has not been shown to have an effect on mortality when compared to aspirin. Warfarin plus aspirin is not recommended for symptomatic PAD.15
Statin Therapy: PAD is considered a cardiovascular risk equivalent, even in asymptomatic patients, according to the ACCF/AHA 2011 guideline. The previous low-density lipoprotein (LDL) goal of 100 mg/dL was based on the treatment recommendations of the National Cholesterol Education Program Adult Treatment Panel III guidelines.19 Although the 2013 release of the ACCF/AHA risk estimator for treatment of blood cholesterol does not consider the presence of PAD, it does mention patients with an ABI <0.9 as a population that may benefit from statin use, although the benefit is less clear.20 In addition to lipid lowering and cardiovascular benefit, statin therapy can modestly improve claudication symptoms and walking distance.4
Ramipril : The angiotensin-converting enzyme (ACE) inhibitor ramipril may also benefit patients with symptomatic PAD. Although studies are limited, one randomized controlled trial of ramipril 10 mg daily found a 77% increase in pain-free walking and a 123% increase in maximum walking times compared to placebo at 6 months. Further studies are needed to determine its place in therapy.21
Surgical Referral
Revascularization can be considered in patients with lifestyle-limiting intermittent claudication who are not achieving adequate relief with exercise and pharmacologic treatment. Revascularization procedures include thrombolysis, endovascular, or surgical. A number of factors determine the type of revascularization that is performed, including site and extent of occlusion, embolus versus thrombus, patient comorbidities, and contraindications to thrombolysis or surgery.9
Up to 1% to 2% of patients progress to critical limb ischemia over 5 years.4 Acute limb ischemia is characterized by rapid or sudden decrease in limb perfusion that threatens tissue viability, most commonly caused by embolism and thrombosis. If done within 14 days of symptom presentation, catheter-based thrombolysis is considered effective and beneficial therapy and is therefore recommended.9
Conclusion
PAD is a prevalent health concern in which pharmacists make a significant contribution in the education and management of patients. Smoking cessation and pharmacologic therapy both play a critical role in improving symptoms and reducing disease progression. The pharmacist should have adequate knowledge of appropriate antiplatelet therapy to optimize patient care.
REFERENCES
1. Libby P. Chapter 241.
The pathogenesis, prevention, and treatment of atherosclerosis. In: Longo DL,
Fauci AS, Kasper DL, et al,
eds.
Harrison’s Principles of Internal Medicine. 18th
ed. New York, NY: McGraw-Hill; 2012.
2.
Criqui MH,
Denenberg JO, Langer RD, et al. The epidemiology of peripheral arterial disease: importance of identifying the population at risk.
Vasc Med. 1997
;2(3):221-226.
3.
Fowkes FG,
Housley E,
Cawood EH, et al. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population.
Int
J
Epidemiol. 1991
;20(2):384-392.
4.
Hennion DR,
Siano KA.
Diagnosis and treatment of peripheral arterial disease.
Am
Fam Physician. 2013
;88(5):306-310.
5.
Griendling KK, Harrison DG, Alexander R. Chapter 8.
Biology of the vessel Wall. In:
Fuster V, Walsh RA, Harrington RA,
eds.
Hurst’s The Heart. 13th
ed. New York, NY: McGraw-Hill; 2011.
6.
Wennberg PW,
Rooke TW. Chapter 109.
Diagnosis and management of diseases of the peripheral arteries and veins. In:
Fuster V, Walsh RA, Harrington RA,
eds.
Hurst’s The Heart. 13th
ed. New York, NY: McGraw-Hill; 2011.
7. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J
Respir
Crit Care Med. 2002
;166(1):111-117.
8. Moyer VA. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013
;159:342-348.
9. American College of Cardiology Foundation/American Heart Association. ACCF/AHA Pocket Guideline November 2011: Management of Patients With Peripheral Artery Disease.
https://www.cardiosource.org/~/media/Files/Science%20and%20Quality/Guidelines/Pocket%20Guides/2011_PAD_PktGuide.ashx. Accessed March 24, 2014.
10. Hamburg NM,
Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation. 2011
;123(1):87-97.
11. Watson L, Ellis B,
Leng GC. Exercise for intermittent claudication. Cochrane Database
Syst Rev. 2008;(4)
:CD000990.
12. Stewart KJ, Hiatt WR,
Regensteiner JG, Hirsch AT. Medical progress: exercise training for claudication. N
Engl J Med. 2002
;347(24):1941-1951.
13. McDermott MM,
Ades P,
Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009
;301:165-174.
14. Prevention and treatment of PAD. Smoking cessation.
American Heart Association.
www.heart.org/HEARTORG/Conditions/More/PeripheralArteryDisease/Prevention-and-Treatment-of-PAD_UCM_301308_Article.jsp. Accessed March 31, 2014.
15.
Alonso-Coello P,
Bellmunt S,
McGorrian C, et al. Antithrombotic therapy in peripheral artery disease. Antithrombotic therapy and prevention of thrombosis, 9th
ed: American College of Chest Physicians Evidence Based Clinical Practice Guidelines. Chest. 2012
;141(2 suppl):e669S-e690S.
16.
Cilostazol.
Lexicomp Online.
www.crlonline.com/lco/action/doc/retrieve/docid/patch_f/6616. Accessed March 19, 2014.
17. Dawson DL, Cutler BS, Hiatt WR, et al.
A comparison of
cilostazol and
pentoxifylline for treating intermittent claudication. Am J Med. 2000
;109(7):523-530.
18. Antithrombotic
Trialists' (ATT) Collaboration,
Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from
randomised trials. Lancet. 2009
;373:1849-1860.
19. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Pressure in Adults (Adult Treatment Panel III) final report: table II.2-3. September 2002.
www.nhlbi.nih.gov/guidelines/cholesterol. Accessed March 31, 2014.
20. Stone NJ, Robinson J, Lichtenstein AH. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation.
2013 Nov 7
[
Epub ahead of print].
21.
Ahimastos AA, Walker PJ, Askew C, et al. Effect of
ramipril on walking times and quality of life among patients with peripheral artery disease and intermittent claudication: a randomized controlled trial. JAMA. 2013
;309
(5):453-460.





