US Pharm.
2006;11:40-47.
Major
depressive disorder (MDD) is a condition that is associated with a sad and
depressed mood. In children, this same mood is accompanied by additional
irritability and hostility. There are limited treatment options for children
with this disorder. Clinicians generally treat children with MDD with the same
medications that are used to treat adults, especially the selective serotonin
reuptake inhibitors (SSRIs). However, concerns exist regarding the possible
link between suicidality (suicidal thoughts and behaviors) and SSRIs and other
antidepressants. Two major reports and a thorough FDA review have addressed
this issue, resulting in significant changes to warnings and labeling for
these medications.
Epidemiology
From 1987 to 1996,
the use of antidepressants increased from 0.3 to 1 per 100 children and
adolescents.1 Specifically, the use of antidepressants increased
from 0.5 (95% CI, 0.19 – 0.81) to 2.1 (95% CI, 1.26 – 2.98) per 100
adolescents ages 15 to 18 during this period.1 The World Health
Organization reported in 2001 that the United States had a 5.3% decrease in
suicide rates2; however, this rate did not distinguish between
age-groups. In February 2004, David Shaffer, MD, from Columbia Uni versity
and the New York State Psychiatric Institute reported in an FDA hearing that
the third leading cause of death in U.S. youths ages 15 to 19 was suicide,
with 1,611 deaths in 2001.3 While depression is the most common
mental disorder that leads to suicide, the issue of whether drug treatment
leads to suicidal behavior in young people is under investigation.2
George Washington
University Medical Center Report
In January 2004,
the George Washington University Medical Center's Center for Health Services
Research and Policy released a Drug Safety Research Special Report on the six
most commonly prescribed antidepressant drugs (i.e., sertraline, paroxetine,
fluoxetine, citalopram, amfebutamone [bupropion], and venlafaxine) and
suicidal/aggressive behaviors in children and adults.4 The center
received a majority of its information from FDA adverse event reports.4
This report concluded that
suicidal and aggressive behaviors were described more often than other "less
serious adverse events" (mania and euphoria) in children.4
Within the year prior to the report, there had been an unusually high number
of reports of suicidal and hostile behaviors in children taking paroxetine and
venlafaxine.4 Although these reports are concerning, it is
important to remember that participation in FDA's reporting system is
voluntary for consumers and health professionals; thus, only a limited number
of events are reported to the FDA.4
The center also concluded that
there are two confounding factors that relate to children and not to adults.
4 First, parents monitor their children's behavior and maintain
oversight. Second, children normally exhibit significant mood swings. There
are insufficient data to assess the effects of these two factors on the
reported outcomes in children and young adults. The increase in suicidal and
aggressive behaviors may simply be due to the increasing number of
antidepressants used in children.
American College of
Neuropsychopharmacology Preliminary Report
The American
College of Neuropsychopharmacology (ACNP) Task Force on SSRIs and Suicidal
Behavior in Youth recently evaluated the safety and efficacy of SSRIs in
children.5 The ACNP was concerned about the role of SSRIs in
suicidal thinking or in suicide attempts in children. The ACNP stated that its
report is only preliminary, as regulatory agencies and pharmaceutical
companies have withheld access to some information.
The ACNP Task Force assessed
results from 15 double-blind, randomized, placebo-controlled clinical trials
that evaluated the effectiveness of SSRIs and other antidepressants in 2,000
children.5 Since many of the trials used more than one outcome
measure, there were multiple interpretations of the results in several of
these studies.
The ACNP focused on two major
studies due to their size and methodology. In the first, 376 patients treated
with sertraline showed improvement in depression symptoms, based on the
Children's Depression Rating Scale–Revised (CDRS-R) total score.
6 Sixty-nine percent of these patients, ages 6 to 17, responded to
treatment, compared to 59% taking placebo (P = .05). Sertraline
produced considerable improvement in the treatment of depression. Adverse
events reported were diarrhea, vomiting, anorexia, and agitation, with other
serious adverse events seen in seven patients in the sertraline group and six
in the placebo group. Also reported in the sertraline group were two suicide
attempts, three suicidal ideations, one aggressive reaction, and one medical
hospitalization. The placebo group had two suicidal ideations and four medical
hospitalizations. The study reported that there was no significant difference
between the sertraline and placebo groups in regard to "other serious adverse
events."
The other major study included
in the ACNP report evaluated the use of fluoxetine for treatment of depression
in children.7 This study by Emslie et al. reported a 41%
improvement in the treatment group compared to 20% in the placebo group (P
<.01) on the CDRS-R.7 The main adverse event reported was
headaches. There were no serious adverse events such as suicidal thinking or
suicide attempts.
The ACNP stated that SSRIs are
the only drugs that should be used to treat depression in children and
adolescents, as other classes of drugs, such as tricyclic antidepressants,
have not been approved for this indication.5 The ACNP report
concluded that there is evidence of efficacy of SSRIs to treat depression in
children.5 Regarding the reports of an association of SSRIs with
suicidal ideations and attempts, first raised about 10 years ago, the ACNP
reported that it did not find a statistically significant relationship between
antidepressants and suicidal behavior or ideation in youth in any of the
studies it considered. It also found that none of the 2,000 young people it
studied died of suicide. The Task Force concluded that claims that SSRIs cause
suicidal behaviors are insufficient without further controlled studies. The
ACNP further concluded that the benefits of treating depression with SSRIs
outweigh the risks of suicidal behaviors.5
FDA Hearings and Reports
In 2003, the FDA issued a Public
Health Advisory to warn physicians about reports of antidepressant medications
causing suicidal behaviors in clinical studies of young patients with MDD.
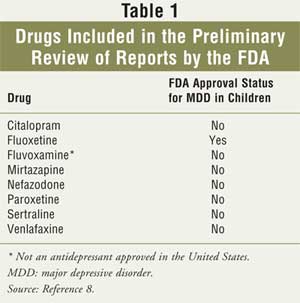
8 Eight drugs (see Table 1) and 4,100 pediatric patients had been
studied to determine if there were any links between these drugs and the
reported increase in suicidal behaviors.9 In October 2003, the FDA
identified 109 cases that were possibly suicide-related, although no deaths
were reported. The agency concluded that there was no direct evidence of a
link between these drugs and suicidality.8 In January 2004, the FDA
issued a statement that no deaths by suicide have been found in young patients
taking antidepressants.9 According to the FDA, adverse events are
difficult to determine when there is no control group, and suicidal thinking
and attempts can also take place in individuals with untreated depression.
9
In February 2004, an FDA
advisory panel recommended that stronger warnings should be included on SSRI
labels.10 The panel also concluded that more controlled studies of
antidepressants need to be conducted in children.
This issue was regularly
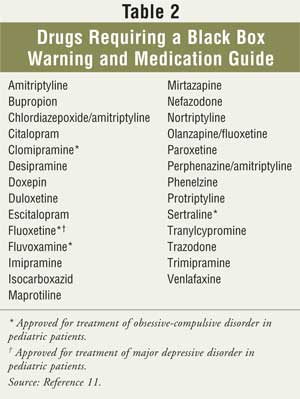
reviewed until the FDA decided October 15, 2004, that all manufacturers of
antidepressants must add to their product labeling a black box warning (see
Table 2 and sidebar), additional statements of warning to
practitioners, and information about pediatric studies.11 The FDA
reached its decision after reviewing data from a combined analysis of 24
trials that included more than 4,400 pediatric patients and nine
antidepressants (the eight drugs in Table 1 and bupropion). The
analysis revealed a 4% average risk of suicidality in patients taking these
medications, which was twice the 2% risk found in the placebo group. Although
no suicides occurred in either group, the FDA determined that antidepressants
do increase the risk of suicidality; thus, stronger warnings for health care
providers, patients, and caregivers were warranted.11 In addition
to the changes in the package insert, the FDA also required that a Patient
Medication Guide be given to patients to advise them of the suicidality risk
and precautions that should be taken.11

Pharmacist Counseling
As health care
professionals, pharmacists may be asked questions regarding this sensitive
issue. The FDA recommends at this time that parents or guardians of pediatric
patients be advised to consult with their physicians before having their
children discontinue antidepressants.8 It is important to counsel
on this since many antidepressant medications should not be stopped suddenly.
8 Furthermore, pharmacists should closely monitor patients with a high
risk of suicidal or aggressive behaviors during initiation of drug therapy and
counsel parents to monitor children for suicidal ideation and behaviors during
drug initiation and discontinuation and when there are dosage changes.8,11
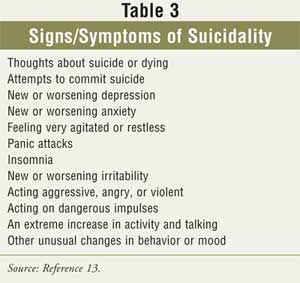
Any behavioral changes relating to suicidality (Table 3) should be
reported to the prescriber immediately.11 Patients and caregivers
should be informed that close observation by the prescriber is also suggested.
The FDA recommends at least a weekly visit for the first four weeks of
treatment as well as when a change in dosage is made. This should be followed
by a visit every other week for the next four weeks, a visit at 12 weeks, and
regular visits as necessary.12 Ultimately, physicians and parents
need to determine if the benefits outweigh the risks of treatment.
Conclusion
There are several possible reasons
for suicidal behaviors, including the nature of depression itself. It is also
possible that other medications, aside from antidepressants, could trigger
these behaviors or add to the effect of the antidepressants. A confounding
factor in interpreting studies on this subject is that they do not clearly
differentiate between "child" or "young adult."
The FDA, as well as George
Washington University Medical Center's Center for Health Services Research and
Policy and the ACNP, has concluded that more clinical trials are needed before
any conclusions can be drawn regarding the relationship between
antidepressants and suicidal behaviors in young people. Since studies to date
have evaluated suicidal behaviors only as an adverse event, it is vital that
future research uses suicidal behaviors as the focus. Until then, only
fluoxetine should be used to treat MDD in children, as it is the only SSRI
approved for this age group.8
References
1. Olfson M, Marcus SC, Weissman MM, Jensen PS. National trends in the use of psychotropic medications by children. J Am Acad Child Adolesc Psychiatry. 2002;41:514-521.
2. The World Health Report 2001. Suicide. Available at: www.who.int/whr2001/2001/main/en/chapter2/002g.htm. Accessed February 23, 2004.
3. Shaffer D. Suicide and Related Problems in Adolescence. Presented at the FDA meeting, February 2, 2004; Washington, D.C.
4. Moore TJ. Drug Safety Research
Special Report. Antidepressant
Drugs and Suicidal/Aggressive Behaviors. George Washington University Medical
Center, Center for Health Services Research and Policy. January 26, 2004:1-27.
5. Mann JJ, Emslie G, et al. Executive Summary: Preliminary Report of the Task Force on SSRIs and Suicidal Behavior in Youth. American College of Neuropsychopharmacology, Inc. 2004:1-22.
6. Wagner KD, Ambrosini P, Rynn M, et al. Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder. JAMA. 2003;290:1033-1041.
7. Emslie GJ, Heiligenstein JH, Wagner KD, et al. Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2002;41:1205-1215.
8. FDA issues public health advisory entitled: reports of suicidality in pediatric patients being treated with antidepressant medications for major depressive disorder (MDD). FDA Talk Paper: October 27, 2003. Available at: www.fda.gov/bbs/topics/ANSWERS/2003/ANS01256.html. Accessed February 13, 2004.
9. Pediatric antidepressants and suicidality. FDA patient safety news: show #23, January 2004. Available at: www.accessdata.fda.gov/scripts/cdrh/cfdocs/psn/printer.cfm?id=191. Accessed February 13, 2004.
10. Holden C. Psychopharmacology: FDA weighs suicide risk in children on antidepressants. Science. 2004;303:745.
11. Suicidality in children and adolescents being treated with antidepressant medications. FDA public health advisory: October 15, 2004. Available at: www.fda.gov/cder/drug/antidepressants/SSRIPHA200410.htm. Accessed August 18, 2005.
12. Class suicidality labeling language for antidepressants. Labeling template: February 3, 2005. Available at: www.fda.gov/cder/ drug/antidepressants/PI_template.pdf. Accessed August 18, 2005.
13. Medication guide about using antidepressants in children and teenagers. Medication guide template: February 3, 2005. Available at: www.fda.gov/cder/drug/antidepressants/MG_template.pdf. Accessed August 18, 2005.
14. Labeling change request letter
for antidepressant medications. Department of Health and Human Services:
October 15, 2004. Available at:
www.fda.gov/cder/drug/antidepressants/SSRIlabelChange.htm. Accessed August 18,
2005.
To comment on this article, contact
editor@uspharmacist.com.






