ABSTRACT: Patients with hematologic malignancies and hematopoietic stem-cell transplants are at high risk for fatal invasive fungal infections. In this patient subpopulation, fungal infections are commonly caused by Candida and Aspergillus species, and if not managed properly, they can result in significant mortality and morbidity. In order to prevent such infections, prophylactic antifungal drug therapy is recommended for patients with poor immune function. Certain medications from the azole and echinocandin drug classes are considered first-line agents for prophylaxis. While these agents are highly efficacious, pharmacokinetic properties and adverse-effect profiles should be considered for optimal therapeutic outcomes.
US Pharm. 2017;42(Specialty&Oncology suppl):20-23.
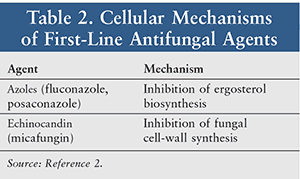
In addition to other health issues, patients with immune-system defects are at high risk for invasive fungal infections. Immunocompromised states in which such fungal infections commonly occur include (Table 1) hematologic malignancies (leukemia, myelodysplastic syndrome); severe neutropenia; mucositis in autologous hematopoietic stem-cell transplants (HSCT); and significant graft-versus-host disease (GVHD).1 If not treated promptly, systemic fungal infections may be fatal.
Among the yeast pathogens, Candida albicans is the most common pathogen, although other non-albicans Candida species are capable of systemic infections as well.1 Yeasts enter the body through the gastrointestinal (GI) tract when the mucosal barrier is disrupted by the toxic insults of chemotherapy and radiation treatment. Subsequently, the pathogens enter the circulation, resulting in candidemia, a serious blood-borne infection.
Inhalation of Aspergillus fumigatus, on the other hand, affects the lung cells and is followed by pneumonia. Other manifestations of Aspergillus infections include sinusitis, cerebral infarction, and skin ulcers.
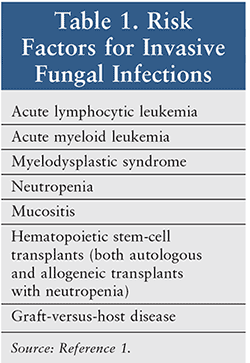
It is currently believed that prophylaxis in immunocompromised patients can markedly reduce the number of deaths and serious complications associated with fungal infections.1 Two azoles (posaconazole, fluconazole) and an echinocandin, micafungin, are the first-line agents for this purpose (see Table 2 for cellular mechanisms of first-line agents).1,2 Other agents recommended (second-line agents) include voriconazole and lipid formulations of amphotericin B.2
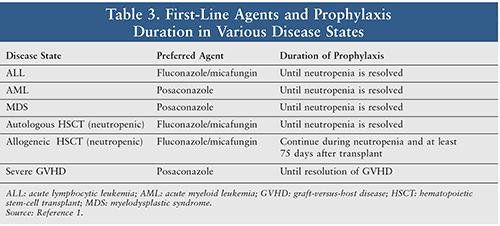
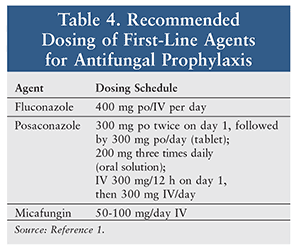
Duration of antifungal prophylaxis varies by disease state (Table 3). Neutropenia (absolute neutrophil count <500 cells/mm3) in certain disease states—such as leukemia—decreases the ability of blood to phagocytose and kill pathogens. Hence, antifungal prophylaxis is continued until the neutrophil count is restored. In severe GVHD, immunosuppressive therapy is needed; this decreases the body’s capacity to fight infections, allowing opportunistic infections to occur.1 A dosing schedule of antifungals for prophylaxis is presented in Table 4. For optimal therapeutic management, other properties of individual therapeutic agents need to be taken into account, as discussed below.
PROPHYLACTIC MEDICATIONS
Important pharmacokinetics, adverse effects, and resistance considerations are listed in the following sections.
First-Line Agents
Fluconazole and Posaconazole: These azoles are generally well tolerated. However, use of both agents is associated with liver toxicity (reversible elevation of liver enzymes) and electrocardiographic abnormality (prolongation of QTc interval).1,2 Compared with other azoles, fluconazole is a weak inhibitor of CYP enzymes, whereas posaconazole is a strong inhibitor of CYP3A4 and a substrate of P-glycoprotein.
For better absorption, posaconazole solution should be taken with a full meal. Proton pump inhibitors decrease plasma levels of posaconazole when administered as an oral solution.1,2
Micafungin: This echinocandin, like other members of this class, does not induce or inhibit CYP enzymes, nor does it interact with P-glycoprotein. As a result, the potential for drug interactions with other therapeutic agents is negligible. Micafungin is well tolerated, and the most common adverse reactions include histamine-mediated effects (rash, pruritus, facial swelling), GI distress, headache, and pyrexia. The drug is a lipopeptide and must not be taken orally.1,2
Second-Line Agents
Voriconazole: This azole, a second-line prophylactic agent, is minimally bound to plasma protein. It is metabolized in the liver by CYP enzymes (CYP3A4, CYP2C9, and CYP2C19) and also inhibits these enzymes. Voriconazole is excreted by the kidney. Transient visual disturbances (blurred vision, changes in color vision or brightness), bone pain, and squamous cell cancer are significant adverse reactions associated with its use.1,2
Amphotericin B: Amphotericin B is a broad-spectrum polyene antifungal agent. Its use is associated with two major adverse effects: infusion-related reactions and renal toxicity. For antifungal prophylaxis, the lipid formulations (liposomal amphotericin B and amphotericin B lipid complex) are used to minimize such adverse reactions.1,2
Treatment Notes
Both voriconazole and posaconazole can increase the serum levels of immunosuppressive agents, including sirolimus and cyclosporine, which may necessitate dose adjustments.2
It is important to note that fluconazole is not active against Aspergillus species, and because of its widespread use, even some Candida species are resistant to its antifungal effects (e.g., Candida krusei).1
IMPORTANT CLINICAL TRIALS
First-Line Agents
Fluconazole: In a double-blind, randomized trial, bone marrow transplant recipients were treated with 400 mg of oral fluconazole daily or placebo from the beginning of the conditioning regimen until their neutrophil count was restored to 1,000/mcl, a toxic reaction was predicted, or a systemic fungal infection was detected.3 Superficial as well as systemic fungal infections occurred significantly less often in fluconazole recipients. With the exception of C krusei, fluconazole treatment prevented infections with other Candida strains. There were fewer fungal infection–related deaths in the fluconazole-treated group (P <.001). Fluconazole treatment caused a mean increase in the liver enzyme alanine aminotransferase; otherwise, the drug therapy was well tolerated.3
In another double-blind, placebo-controlled study, patients who received bone marrow transplants along with prophylactic therapy with fluconazole experienced a decrease in systemic fungal infections.4 During prophylaxis, systemic fungal infections occurred in only 7% of fluconazole-treated patients compared with 18% of placebo-treated patients. Superficial fungal infections, fungal colonization, and empiric antifungal therapy requirements with amphotericin B were also markedly reduced as a result of fluconazole prophylaxis.4 The total number of deaths was lower compared with the placebo group.
Eight-year follow-up data indicate that fluconazole prophylaxis (400 mg/day) for 75 days following allogeneic bone marrow transplantation increased the number of patients who survived compared with the placebo group.5 The overall incidence of invasive candidiasis was lower in patients who received fluconazole prophylaxis (P <.001). The incidence of GVHD in the gut and deaths from complications were also found to be lower in patients receiving fluconazole prophylaxis.
In a multicenter, double-blind trial, patients aged 18 to 80 years of both genders undergoing cytotoxic chemotherapy for leukemia or autologous bone marrow transplant received daily oral treatment of 400 mg fluconazole until neutropenia was resolved for 2 days, parenteral amphotericin B therapy was initiated, or a maximum duration of 60 days was reached.6 Antifungal prophylaxis decreased fungal colonization (P = .004) and invasive fungal infections (P = .0001). Moreover, fungal infection–related deaths were found to be fewer (P = .04) in fluconazole-treated patients. Patients who were treated with a cytarabine-plus-anthracycline combination or who received an autologous stem-cell transplant and were not treated with any colony-stimulating growth factor received the most benefit from this intervention.6
Posaconazole: Cornely and colleagues compared posaconazole prophylaxis with that of fluconazole or itraconazole in neutropenic patients suffering from acute myeloid leukemia (AML) or myelodysplastic syndromes who were receiving induction or reinduction chemotherapy.7 Posaconazole markedly reduced invasive fungal infections during the treatment period compared with the fluconazole or itraconazole arm (2% vs. 8%, P <.001).7 Also, the occurrence of invasive aspergillosis was markedly lower in posaconazole-treated patients (P <.001). Posaconazole recipients had a longer survival period than fluconazole or itraconazole recipients. However, serious adverse events occurred more often in posaconazole-treated patients as opposed to the fluconazole or itraconazole group.7
Micafungin: In a randomized, double-blind, multicenter, phase III trial, micafungin—an echinocandin—was compared with fluconazole for antifungal prophylaxis in patients who underwent hematopoietic stem-cell transplantation. Of the 889 patients enrolled in the trial, 425 patients were given micafungin 50 mg/day (1 mg/kg for patients weighing <50 kg); fluconazole was administered at 400 mg/day (8 mg/kg for patients weighing <50 kg) to the remaining patients.8 Patients received an IV infusion in a volume of 200 mL administered over a 1-hour period. Treatment success was determined to be the absence of systemic fungal infections throughout the entire prophylactic period and the absence of systemic fungal infection until the end of 4-week postprophylactic therapy. The success rate was found to be markedly higher for patients treated with micafungin as opposed to the fluconazole group (80% vs. 73.5%). The most frequent adverse effect observed in both treatment arms was abnormal liver parameters.8
Second-Line Agents
Voriconazole: Voriconazole (200 mg twice daily, orally) was compared with fluconazole (400 mg daily, orally) in a randomized, double-blind trial (N = 600; voriconazole group: 305 patients; fluconazole group: 295 patients) in allogeneic bone-marrow transplant recipients for 100 days (for high-risk patients, prophylaxis was continued for 180 days).9 Absence of invasive fungal infections and absence of fungal infection–related deaths for 6 months were the primary endpoints of the study. Although no difference was noted in the primary endpoints, patients in the voriconazole-treated group displayed a declining trend in Aspergillus infections, invasive fungal infections, and the requirement of empirical antifungal treatment. Adverse drug reactions were equivalent in both treatment groups.9
AML patients (N = 25) receiving remission-induction chemotherapy were treated with oral voriconazole (200 mg twice daily) or placebo in a randomized, double-blind, placebo-controlled, phase III study.10 Primary efficacy was determined based on the appearance of pulmonary infiltrates after day 21 of the initiation of chemotherapy. There was a declining trend of lung infiltrate in voriconazole-treated patients. At 4-week follow-up, hepatosplenic candidiasis was reported in patients belonging to the placebo group; none was reported in the voriconazole-treatment group. The drug therapy was well tolerated by the patients.10
Amphotericin B: A total of 271 patients having hematologic disease with expected neutropenia of ≥10 days were included in a randomized, placebo-controlled trial for liposomal amphotericin B (n = 139) or placebo (n = 132) inhalation on a twice-weekly regimen until the neutrophil count reached >300 cells/mm3 with a maximum of 12 inhalations per neutropenic episode.11 The liposomal amphotericin B dosage was 5 mg/mL in a total volume of 5 mL. Nebulization was carried out for 30 minutes/day for 2 consecutive days per week. There were significantly fewer invasive pulmonary aspergillosis cases in patients who received amphotericin B inhalation. Coughing was the most frequent adverse effect from amphotericin B treatment.11
In an open-label trial, adult patients (N = 48) on induction chemotherapy for AML received a high dosage of liposomal amphotericin B (15 mg/kg by infusion).12 Patients with 15 days of persistent neutropenia were treated with a second dose of the drug. Hypokalemia was reported in six patients, but nephrotoxicity was absent. In one patient, drug therapy was discontinued for infusion-related infections. Only 8.3% of patients developed invasive fungal disease.12
Koh and colleagues compared fluconazole (200 mg/day orally) versus low-dose amphotericin B (0.2 mg/kg/day IV) in patients who underwent allogeneic or autologous HSCT.13 The treatment was started 1 day prior to the beginning of the conditioning regimen and continued until one of the following conditions occurred: engraftment, drug toxicity, or systemic fungal infections. Findings indicated there was no significant difference in fungal infections, survival, or death.13
ROLE OF THE PHARMACIST
Since systemic fungal infections can result in fatalities, pharmacists can play a pivotal role in selecting the appropriate antifungal agent for immunocompromised patients with cancer and stem-cell transplants. Minimizing drug-drug interactions and serious toxicities associated with the use of these agents is equally important for patients’ wellbeing. Another important role of the pharmacist is to periodically monitor organ functions, particularly those of the liver (for azoles) and kidneys (for amphotericin B). Also, as treatment guidelines continue to change, pharmacists need to keep abreast of medical literature to enhance knowledge and competence.
CONCLUSION
This article discussed important therapeutic agents for antifungal prophylaxis in immunocompromised states. According to the current literature, the occurrence of systemic fungal infections can be successfully prevented by proper and timely use of effective antifungal agents. Individualized therapeutic monitoring for drug efficacy and safety are equally important parameters for successful treatment outcomes. Clinical pharmacists have been instrumental in fulfilling such crucial tasks.
REFERENCES
1. Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:882-913.
2. Sheppard D, Lampiris HW. Antifungal agents. In: Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology. 11th ed. New York, NY: McGraw-Hill Medical; 2009:835-844.
3. Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845-851.
4. Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation—a prospective, randomized, double-blind study. J Infect Dis. 1995;171:1545-1552.
5. Marr KA, Seidel K, Slavin MA, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood. 2000;96: 2055-2061.
6. Rotstein C, Bow EJ, Laverdiere M, et al. Randomized placebo-controlled trial of fluconazole prophylaxis for neutropenic cancer patients: benefit based on purpose and intensity of cytotoxic therapy. The Canadian Fluconazole Prophylaxis Study Group. Clin Infect Dis. 1999;28:331-340.
7. Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348-359.
8. van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39:1407-1416.
9. Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116:5111-5118.
10. Vehreschild JJ, Böhme A, Buchheidt D, et al. A double-blind trial on prophylactic voriconazole (VRC) or placebo during induction chemotherapy for acute myelogenous leukaemia (AML). J Infect. 2007;55:445-449.
11. Rijnders BJ, Cornelissen JJ, Slobbe L, et al. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial. Clin Infect Dis. 2008;46:1401-1408.
12. Annino L, Chierichini A, Anaclerico B, et al. Prospective phase II single-center study of the safety of a single very high dose of liposomal amphotericin B for antifungal prophylaxis in patients with acute myeloid leukemia. Antimicrob Agents Chemother. 2013;57:2596-2602.
13. Koh LP, Kurup A, Goh YT, et al. Randomized trial of fluconazole versus low-dose amphotericin B in prophylaxis against fungal infections in patients undergoing hematopoietic stem cell transplantation. Am J Hematol. 2002;71:260-267.
To comment on this article, contact rdavidson@uspharmacist.com.