Coronary heart disease (CHD) is a very prevalent medical condition, with estimated direct and indirect costs for 2009 of $165.4 billion.1 It is projected that 785,000 Americans will have a new coronary attack and over 470,000 will have a recurrent attack this year. In 2005, one out of every five deaths was caused by CHD.1
Acute coronary syndrome (ACS), a manifestation of CHD, is a spectrum of ailments that encompasses unstable angina (UA) and acute myocardial infarction (MI), with or without ST-segment elevation (STEMI or NSTEMI). ACS is the end result of atherosclerosis, a diffuse disease of medium- and large-sized arteries characterized by endothelial dysfunction, atherogenesis, and plaque formation.2 Platelets play a fundamental role in the pathophysiology of atherosclerosis. Platelet adhesion to a damaged vessel wall is the first step in arterial thrombosis formation. Activation of platelets involves a morphological change in the platelet to increase aggregation; this occurs through various mechanisms involving adenosine diphosphate (ADP) and thromboxane A2, both of which are targets for antiplatelet therapy (i.e., aspirin [ASA], thienopyridines).
Initial management of patients with ACS can take either a conservative medical management approach or an early invasive strategy depending on the presentation.3-5 Medical management may include therapy with anticoagulation (e.g., heparin, enoxaparin), glycoprotein IIb/IIIa inhibitors (e.g., eptifibatide, tirofiban, abciximab), and/or fibrinolytics (e.g., alteplase, tenecteplace). The primary difference with invasive management of ACS is that it includes percutaneous coronary intervention (PCI), in which an intracoronary stent is placed to restore blood flow to the compromised myocardium. In 2006, over 1.3 million PCIs were performed in the United States.1 Intracoronary stents are produced in two forms: bare metal stents (BMS) and drug-eluting stents (DES). During the time frame 2004 to 2005, over 80% of the intracoronary stents placed were of the DES variety.6 DES have the same structure as BMS but are coated with an antiproliferative medication. There are currently four DES available in the United States: Taxus (paclitaxel), Cypher (sirolimus), Xience (everolimus), and Endeavor (zotarolimus).7-10
Two main issues of concern with intracoronary stents are the development of restenosis and the phenomenon of stent thrombosis. Restenosis, which occurs primarily with BMS, is a gradual return of the narrowing of the vessel, manifested by the return of angina symptoms.11
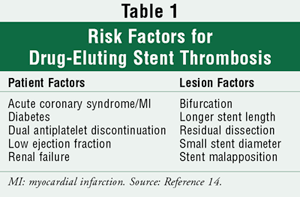
Stent thrombosis is typically a catastrophic event where patients experience UA, nonfatal MI, or death.12 In a nine-month follow-up of patients who underwent DES implantation, 7% of the cases of stent thrombosis presented as UA, 60% as nonfatal MI, and 24% as death.13 Stent thrombosis is defined by the time course of the occurrence. A recently proposed definition is as follows: acute within 24 hours, subacute in 24 hours to 30 days, late after 30 days, and very late after 12 months.14 Acute and subacute thromboses have occurred in both BMS and DES. Late and very late stent thromboses, while as a rule a rare finding (<1%), occur primarily with DES.15 There have been several predictors identified for DES thrombosis (TABLE 1). In one study, the premature discontinuation of antiplatelet therapy resulted in a 29% incidence of stent thrombosis.13 This resulted in a hazard ratio (HR) of 161 (95% confidence interval [CI], 26-998) for subacute stent thrombosis and an HR of 57 (95% CI, 15-220) for late stent thrombosis.13
Secondary prevention of ACS takes a multifaceted approach that encompasses many measures directed at risk factor modification.16 Risk factor modification is accomplished through optimal control of comorbid conditions (i.e., diabetes, hypertension, hyperlipidemia), as well as lifestyle modifications (e.g., smoking cessation, increased physical activity, and weight loss). Another measure for secondary prevention includes maximizing medical therapy with angiotensin-converting enzyme inhibitors, beta-adrenergic antagonists, HMG-CoA reductase inhibitors, and antiplatelet agents.16 Dual antiplatelet therapy (DAT) has taken a prominent role in secondary prevention based on its documented benefit over monotherapy.
DAT is accomplished by using medications that affect platelet functions through different mechanisms. The medications most commonly used are ASA and the thienopyridines (e.g., clopidogrel, ticlopidine). ASA exerts its antiplatelet activity through inhibition of cyclooxygenase and reduction of thromboxane A2.17 Primary concerns with ASA therapy include bleeding and gastrointestinal (GI) toxicity. Generalized bleeding with ASA is uncommon with monotherapy in the absence of underlying hemostatic defect (e.g., hemophilia or anticoagulation therapy).17 The risk of GI side effects (e.g., bleeding, ulcers) is increased two- to four-fold with the use of ASA and appears to be dose related in the range of 30 to 1,300 mg/day.17,18
Thienopyridines (clopidogrel and ticlopidine) exert antiplatelet activity through action at the P2Y12 receptor resulting in a reduction in ADP-induced platelet aggregation.17 The risks of therapy differ between the thienopyridines. Both drugs have a risk of bleeding and GI toxicity that is equal to or possibly less than that of ASA.17,18 Ticlopidine has an effect on the bone marrow that results in a risk of thrombotic thrombocytopenia purpura (TTP) reported to be 0.02%.17 It is recommended to monitor a complete blood count with differential every two weeks during the first three months of therapy with ticlopidine. The incidence of TTP is lower with clopidogrel and, therefore, clopidogrel is the thienopyridine of choice.17
This article will review the guidelines for and evidence supporting the use of DAT for the secondary prevention of ACS, whether managed invasively or conservatively. It will also highlight the ways pharmacists can improve adherence with these recommendations.
Guideline Recommendations
There have been a number of guidelines published by the American Heart Association (AHA), American College of Cardiology (ACC), Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association that address the dosage and duration of DAT needed for the secondary prevention of ACS.3-5,19,20 These recommendations will be summarized in the following sections.
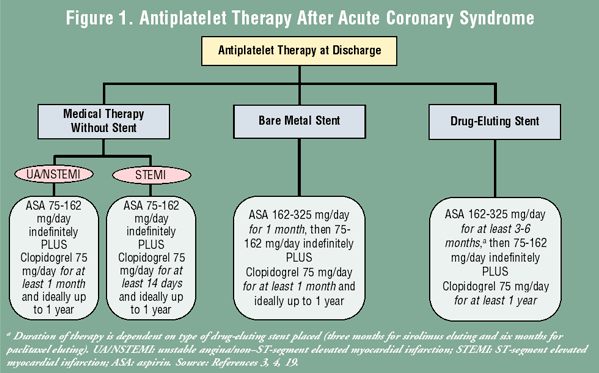
The decision on the dosage and duration of DAT is driven by the patient’s presentation (described in the following sections) and the type of intervention the patient receives (medical management vs. intervention). When the patient requires PCI, the duration of DAT is based on the type of stent placed (BMS vs. DES) (FIGURE 1). There are several large, randomized controlled studies that demonstrate the effectiveness of DAT in these various patient populations (TABLE 2).
Unstable Angina/Non–ST-Segment Elevated MI (UA/NSTEMI): For patients presenting with UA/NSTEMI who are being medically managed, the recommendation is to continue ASA at a dose of 75 to 162 mg/day indefinitely in combination with clopidogrel 75 mg/day for at least one month, but preferably up to one year based on results from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial (TABLE 2).5,21 The CURE trial included 12,562 patients who presented with non–ST-segment elevated ACS who were mostly managed medically (>75% did not receive a PCI).21 Patients were randomized to receive clopidogrel 75 mg daily in addition to ASA 75 to 325 mg daily, or ASA 75 to 325 mg daily with placebo for up to 12 months. The primary end point of death from cardiovascular causes, nonfatal MI, or stroke was significantly reduced with the use of clopidogrel versus placebo (relative risk [RR] 0.80; 95% CI, 0.72-0.90; P <.001).21 This result was primarily driven by a reduction in nonfatal MI (RR 0.77; 95% CI, 0.67-0.89; P <.05).21
An additional trial regarding the use of DAT in high-risk patients is the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study (TABLE 2).22,23 This investigation was designed to examine the effects of long-term DAT in a broad patient population. Bhatt et al enrolled 15,603 patients with either clinically evident cardiovascular disease or multiple risk factors for cardiovascular disease.22,23 Patients were randomized to receive either ASA 75 mg to 162 mg daily plus clopidogrel 75 mg daily or ASA 75 mg to 162 mg daily plus placebo and were followed for a median duration of 28 months.22,23 The addition of clopidogrel to low-dose ASA showed a nonsignificant reduction in the primary end point of MI, stroke, or death from cardiovascular causes (6.8% vs. 7.3%; RR 0.93; 95% CI, 0.83-1.05; P =.22).22 There was a significant reduction in one component of the combined end point, nonfatal stroke (RR 0.79; 95% CI, 0.64-0.98; P =.003).22 However, the CHARISMA study was designed using a combined patient population (some patients were receiving primary prevention while others were receiving secondary prevention), making it difficult to interpret the results. Generally, this study is interpreted as a reason not to use DAT in primary prevention.24
To further investigate the use of DAT for secondary prevention, a subgroup analysis of the CHARISMA trial was performed (TABLE 2). This included patients in the CHARISMA trial who did have documented atherothrombotic disease (i.e., previous MI, stroke, or symptomatic peripheral arterial disease).25 This was a robust subgroup containing 9,478 patients. In this analysis, authors confirmed a 17% relative risk reduction (RRR) in cardiovascular death, MI, or stroke with clopidogrel versus placebo (7.3% vs. 8.8%; HR 0.83; 95% CI, 0.72-0.96; P =.01).25 These findings reinforced the evidence that DAT is efficacious for the secondary prevention of cardiovascular disease.
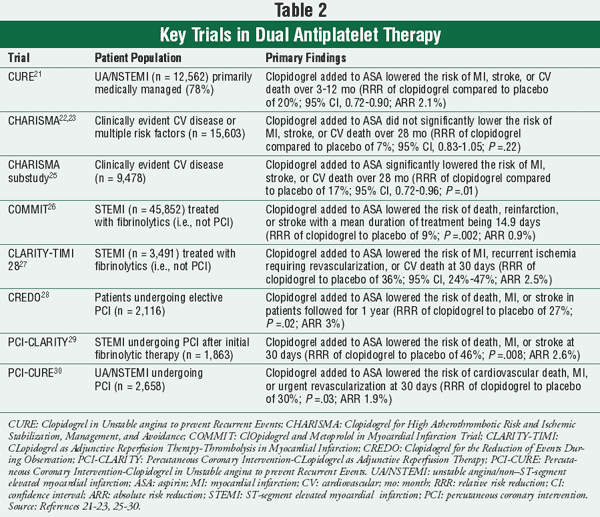
ST-Segment Elevated MI (STEMI): The most recent update to the STEMI guidelines includes a new recommendation: the addition of clopidogrel 75 mg daily plus ASA 75 mg to 162 mg daily in all patients with STEMI, whether or not they undergo reperfusion therapy.4 Treatment with clopidogrel should continue for at least 14 days, and ASA should continue indefinitely.4 This recommendation is based on the results from two trials, the ClOpidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT) and the CLopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis in Myocardial Infarction 28 (CLARITY-TIMI 28) study (TABLE 2).26,27
COMMIT included 45,852 patients with suspected MI (93% met criteria for STEMI and 54% were treated with fibrinolytics).26 Patients were randomized to receive either placebo plus ASA 162 mg daily or clopidogrel 75 mg daily plus ASA 162 mg daily. Clopidogrel was continued for a mean duration of 14.9 days after randomization. The primary end point of death, reinfarction, or stroke was significantly reduced in the group receiving clopidogrel versus those receiving placebo at 28 days after randomization (9.2% vs. 10.1%; RRR 9%; P =.002).
The CLARITY-TIMI 28 trial studied 3,491 patients presenting with STEMI who were treated with fibrinolytics.27 Patients were randomized to receive either clopid ogrel (given as a 300 mg load, then 75 mg daily) with ASA 75 mg to 162 mg daily or placebo in addition to ASA 75 mg to 162 mg daily for 30 days. The addition of clopidogrel significantly reduced the primary end point of MI, recurrent ischemia requiring revascularization, or cardiovascular death versus placebo (15.0 % vs. 21.7%; RRR 36%; 95% CI, 24%-47%; P =.001).
Percutaneous Coronary Intervention: The dosing for DAT after PCI is determined primarily by the type of intracoronary stent that is placed and does not differ between UA/NSTEMI and STEMI. The recommendations are based on the antiplatelet regimens used by the stent manufacturers to obtain FDA approval for the stent, as well as the estimated time it takes for the metal stent struts to become endothelialized. Endothelialization, the body’s process of incorporating the stent into the vessel wall, is believed to reduce the risk of stent thrombosis.20 BMS require a dose of ASA 162 mg to 325 mg daily for one month then 75 mg to 162 mg daily indefinitely in combination with clopidogrel 75 mg daily for at least one month, ideally for 12 months.19 DES, which take longer to endothelialize, require a dose of ASA 162 mg to 325 mg daily for three months following a sirolimus DES or six months following a paclitaxel DES, then 75 mg to 162 mg daily indefinitely in combination with clopidogrel 75 mg daily for a minimum of one year.19
The current guidelines do not address the newer DES, but it is reasonable to continue an increased dose of ASA (162-325 mg daily) for a short time after implantation, as well as clopidogrel for one year. These recommendations are derived from three main trials: the Clopidogrel for the Reduction of Events During Observation (CREDO), Percutaneous Coronary Intervention-CLopidogrel as Adjunctive Reperfusion Therapy (PCI-CLARITY), and Percutaneous Coronary Intervention-Clopidogrel in Unstable angina to prevent Recurrent Events (PCI-CURE) trials (TABLE 2).28-30
The CREDO trial examined 2,116 patients undergoing elective coronary catheterization with planned PCI or high likelihood of PCI.28 The study did not specify what type of stent was used (i.e., BMS or DES). Patients were randomized to receive a 300-mg loading dose of clopido grel or placebo, then both groups continued clopidogrel 75 mg daily and ASA 325 mg daily until day 28. Both groups continued to receive standard ASA therapy (81-325 mg daily) for one year. On day 29, the group that did not receive the loading dose of clopidogrel was changed to placebo, while the other continued clopidogrel 75 mg daily. The continuation of DAT for one year resulted in a 26.9% RRR in the composite end point of death, MI, or stroke (8.5% vs. 11.5%; 95% CI, 3.9%-44.4%; P =.02).
The PCI-CLARITY trial studied the subset of patients in CLARITY-TIMI 28 who proceeded to PCI.29 All patients in the CLARITY-TIMI 28 study were mandated to have coronary angiography two to eight days after randomization.27 If at the time of angiography the patient received an intracoronary stent, the individual was included in the PCI-CLARITY substudy. A total of 53.4% (n = 1,863) of the patients in CLARITY-TIMI 28 ultimately received PCI. At the time of PCI (~3 days after randomization), patients who were originally loaded with placebo were given a loading dose of 300 mg of clopido grel. Both groups (early- and delayed-start clopidogrel) received clopidogrel 75 mg daily in addition to ASA 75 mg to 162 mg daily for 30 days after PCI. Early treatment with clopidogrel resulted in a 46% RRR for cardiovascular death, MI, or stroke following PCI versus delayed treatment (3.6% vs. 6.2%; odds ratio [OR] 0.54; 95% CI, 0.35-0.85; P =.008).29
The PCI-CURE trial examined the subset (n = 2,658) of patients in the CURE trial who proceeded to have PCI.30 Both groups took an open-label thienopyridine (clopidogrel or ticlopidine) for two to four weeks following PCI and then returned to randomly assigned studied medication (clopidogrel 75 mg daily plus ASA 75-325 mg daily or placebo plus ASA 75-325 mg daily) for the remainder of the study (mean of 8 months). The continuation of clopidogrel with ASA showed a 30% RRR in cardiovascular death, MI, and urgent revascularization versus placebo and ASA at 30 days (4.5% vs. 6.4%; RR 0.7; 95% CI, 0.5-0.97; P =.03). There was also a 17% RRR demonstrated in the same end point continued to the end of follow-up (~8 months) (18.3% vs. 21.7%; RR 0.83; 95% CI, 0.7-0.99; P =.03).30
Precautions
DAT is highly efficacious in reducing morbidity and mortality for the secondary prevention of ACS in a variety of patient subsets. However, these benefits do not come without a cost. DAT carries a significantly higher risk of bleeding compared to the use of ASA alone. In the two studies with the longest follow-up, CURE (~1 year) and CHARISMA (28 months), there was a substantial increase in the incidence of minor to severe bleeding with the use of DAT. CURE demonstrated a statistically significant increase in both minor (RR 2.12; 95% CI, 1.75-2.56; P <.001) and major bleeding (RR 1.38; 95% CI, 1.13-1.67; P =.001).21 CHARISMA showed mixed results in the use of DAT, with a increase in moderate bleeding (RR 1.62; 95% CI, 1.27-2.08; P <.001) and a trend toward an increase in severe bleeding (RR 1.25; 95% CI, 0.97-1.61; P =.09).22 However, in the CHARISMA subset, there was no increased risk of moderate or severe bleeding after one year with DAT in patients who had tolerated therapy for one year without bleeding.24
The risks of bleeding are magnified in the presence of coronary surgery. This is reflected in the guidelines by the recommendation that clopidogrel be held for at least five days prior to coronary artery bypass graft surgery.4,5,16 Nevertheless, this therapy is lifesaving, and the increased risk of bleeding does not outweigh the overwhelming benefit seen with DAT.
Other medications that increase the risk of bleeding must be carefully managed while patients are on DAT. Concomitant anticoagulation with warfarin significantly increases the risk of bleeding, and it is recommended to reduce the target international normalized ratio (INR) to 2.0 to 2.5.18 It is also recommended that concomitant therapy with nonsteroidal anti-inflammatory drugs be avoided. DAT should be used in caution with patients with a history of peptic ulcer disease due to the higher risk of bleeding. These patients should be managed with concomitant proton pump inhibitors (PPIs) to minimize this risk.18
Pharmacist’s Role
The pharmacist can have a number of positive impacts on the utilization of DAT. An initial step would be to intervene in an attempt to increase employment of this therapy at hospital discharge. In the face of overwhelming evidence of benefit, DAT is not routinely continued at hospital discharge based on the finding of the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines (CRUSADE) registry.31 Peterson et al examined data from 350 academic and nonacademic U.S. hospitals, which included 64,775 patients enrolled in the CRUSADE registry. Their findings revealed that while 90% of patients were discharged on ASA, only 54% were discharged on clopidogrel.31 All of them were non–ST-segment elevated ACS patients who would have qualified for at least a month (if not one year) of DAT.
A second area for pharmacists to improve the utilization of this therapy is through efforts to increase patients’ adherence and prevent premature discontinuation. In a recent study, Spertus et al reported that 13.6% of patients who had received a DES are no longer taking a thienopyridine at one month.32 When these patients were followed out to one year, they had a 10-fold increase in risk of death (7.5% vs. 0.7%, P <.001). The authors identified a number of factors present in the patients who prematurely discontinued the thienopyridines. These patients were older (64 vs. 60 years, P =.03), less likely to have finished high school, less likely to be married, more likely to avoid health care due to cost, and more likely to have preexisting cardiovascular disease or anemia. The early discontinuation group also included patients who were less likely to have been given discharge instructions regarding medications and who were less likely to have been referred to cardiac rehabilitation. The authors concluded that these results reveal a distinct need for additional patient education regarding the importance of continuation of DAT, especially in patients with less formal education. This is a prime opportunity for pharmacists to intervene on our patients’ behalf. Patients can benefit from additional education on the importance of adherence to DAT therapy, the proper duration, and the risks of therapy. Pharmacists are in a key position to increase patients’ knowledge.
Another risk of premature discontinuation of DAT may be due to the recommendation of the patient’s family physician, dentist, or other health care provider who does not have a complete understanding of the risk-to-benefit ratio of this therapy. Generally, patients should be counseled not to stop DAT without consulting with their treating cardiologist.20 Elective procedures where there is a significant risk of bleeding should be postponed until the completion of DAT (i.e., one year for DES and one month for BMS). If a procedure is required and patients must discontinue thienopyridine, ASA should be continued if at all possible and the thienopyridine restarted as soon as possible.
One other identified risk for the nonuse of DAT is the presence of either an ASA or a clopidogrel allergy. An initial approach a pharmacist should take is to confirm the presence of a true allergy versus drug intolerance. A patient with a history of GI distress could be managed with the addition of a PPI.18 Caution must be used when selecting a PPI because there is a drug interaction between omeprazole and clopidogrel that reduces the antiplatelet activity of clopidogrel.33 If a true allergy is present, this should not be viewed as a barrier to DAT. There are a number of published strategies that have been used to successfully desensitize patients to either ASA or clopidogrel.34-38 All of these protocols are accomplished within 24 hours and would allow the administration of optimal therapy.
Conclusion
Dual antiplatelet therapy is an imperative medical therapy for the secondary prevention of ACS. It has been shown to be effective in patients with UA, NSTEMI, or STEMI and in those following PCI. Special attention needs to be paid to the dosage and duration of ASA, as well as clopidogrel in regards to the type of stent placed (i.e., DES or BMS). Pharmacists can have positive impacts on the utilization of this therapy by promoting use at discharge, improving adherence, assessing allergies to medications, and recommending desensitization as appropriate.
REFERENCES
1. Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 Dec 15. Epub ahead of print.
2. Steinhubl SR, Moliterno DJ. The role of the platelet in the pathogenesis of atherothrombosis. Am J Cardiovasc Drugs. 2005;5:399-408.
3. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary. A report of the ACC/AHA Task Force on Practice Guidelines. J Am Coll Cardiol. 2004;44:671-719.
4. Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the ACC/AHA Task Force on Practice Guidelines. Circulation. 2008;117:296-329.
5. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the ACC/AHA Task Force on Practice Guidelines. Circulation. 2007;116:803-877.
6. National Center for Health Statistics. Health, United States, 2007 With Chartbook on Trends in the Health of Americans. Hyattsville, MD: NCHS; 2007.
7. Taxus (paclitaxel-eluting stent) package insert. Natick, MA: Boston Scientific Corporation; 2008.
8. Cypher (sirolimus-eluting stent) package insert. Miami Lakes, FL: Cordis Neurovascular, Inc; 2007.
9. Endeavor (everolimus-eluting stent) package insert. Santa Rosa, CA: Medtronic, Inc; 2008.
10. Xience V (zotarolimus-eluting stent) package insert. Santa Clara, CA: Abbott Vascular; 2008.
11. Bavry AA, Bhatt DL. Appropriate use of drug-eluting stents: balancing the reduction in restenosis with the concern of late thrombosis. Lancet. 2008;371:2134-2143.
12. Jaffe R, Strauss BH. Late and very late thrombosis of drug-eluting stents: evolving concepts and perspectives. J Am Coll Cardiol. 2007;50:119-127.
13. Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126-2130.
14. Hodgson JM, Stone GW, Lincoff AM, et al. Late stent thrombosis: considerations and practical advice for the use of drug-eluting stents: a report from the Society for Cardiovascular Angiography and Interventions Drug-eluting Stent Task Force. Catheter Cardiovasc Interv. 2007;69:327-333.
15. Jeremias A, Kirtane A. Balancing efficacy and safety of drug-eluting stents in patients undergoing percutaneous coronary intervention. Ann Intern Med. 2008;148:234-238.
16. Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the NHLBI. Circulation. 2006;113:2363-2372.
17. Patrono C, Baigent C, Hirsh J, Roth G. Antiplatelet drugs: American College of Chest Physicians evidence-based clinical practice guidelines (8th ed). Chest. 2008;133(suppl):199S-233S.
18. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/ AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the ACC Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008;118:1894-1909.
19. King SB III, Smith SC Jr, Hirshfeld JW Jr, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the ACC/AHA Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:172-209.
20. Grines CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the AHA, ACC, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association. Circulation. 2007;115:813-818.
21. Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494-502.
22. Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706-1717.
23. Bhatt DL, Topol EJ, CHARISMA Executive Committee. Clopidogrel added to aspirin versus aspirin alone in secondary prevention and high-risk primary prevention: rationale and design of the CHARISMA trial. Am Heart J. 2004;148:263-268.
24. Bakhru MR, Bhatt DL. Interpreting the CHARISMA study. What is the role of dual antiplatelet therapy with clopid ogrel and aspirin? Cleve Clin J Med. 2008;75:289-295.
25. Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49:1982-1988.
26. Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute MI: randomised placebo-controlled trial. Lancet. 2005;366:1607-1621.
27. Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352:1179-1189.
28. Steinhubl SR, Berger PB, Mann JT III, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411-2420.
29. Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA. 2005;294:1224-1232.
30. Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527-533.
31. Peterson ED, Roe MT, Mulgund J, et al. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295:1912-1920.
32. Spertus J, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113:2803-2809.
33. Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol. 2008;51:256-260.
34. von Tiehl KF, Price MJ, Valencia R, et al. Clopidogrel desensitization after drug-eluting stent placement. J Am Coll Cardiol. 2007;50:2039-2043.
35. Rossini R, Angiolillo DJ, Musumeci G, et al. Aspirin desensitization in patients undergoing PCIs with stent implantation. Am J Cardiol. 2008;101:786-789.
36. Ramanuja S, Breall JA, Kalaria VG. Approach to “aspirin allergy” in cardiovascular patients. Circulation. 2004;110:e1-e4.
37. Walker NE, Fasano MB, Hobbs RA, Horwitz PA. Clopidogrel desensitization protocol for the treatment of thienopyridine hypersensitivity. Crit Pathw Cardiol. 2007;6:26-29.
38. Page NA, Schroeder WS. Rapid desensitization protocols for patients with cardiovascular disease and aspirin hypersensitivity in an era of dual antiplatelet therapy. Ann Pharmacother. 2007;41:61-67.
To comment on this article, contact rdavidson@jobson.com.





