US Pharm. 2020;45(3):15-19.
ABSTRACT: Chronic pain affects almost one-third of the U.S. population, often negatively impacting activities of daily living. There are a limited number of analgesic medication classes that control pain; one of the most commonly prescribed includes the opioid pain relievers. Some providers are apprehensive about prescribing opioids, and there is interest in medications that do not bind directly to the opioid receptor. One unconventional, nonopioid-based option under study is the atypical antipsychotic class, with a limited number of studies suggesting some evidence for the adjunctive treatment of certain pain disorders, including migraine headaches, chronic pain, neuropathic pain, and fibromyalgia.
Chronic pain can be debilitating and result in the inability to carry out daily functions and a diminished quality of life. It is estimated that over 100 million people in the United States live with chronic pain, almost one-third of the U.S. population.1,2
In the early 1800s to 1900s, pain management primarily consisted of nonpharmacologic measures, with pharmacologic treatment options taking precedence in the mid-1900s.3 By the 1990s, the indication for opioid use had spread to encompass not only cancer-related pain but also acute and chronic noncancer pain.3
Sales of prescription opioids nearly quadrupled from 1999 to 2014.4 Since 1999, the number of drug-overdose deaths due to prescription opioids had been steadily increasing, reaching nearly 72,000 deaths in 2017.3 Prescription pain reliever misuse was the second most common form of illicit drug use in the U.S. in 2018, and it is estimated that 9.9 million people misused opioids in the past year.5
Therefore, many providers are interested in prescribing pain medications that do not bind directly to the opioid receptor for fear of misuse, abuse, addiction, dependence, tolerance, and withdrawal. One class of medications that has been receiving more attention to augment other nonopioid pain medications—acetaminophen, traditional nonsteroidal anti-inflammatories (e.g., naproxen, ibuprofen, and aspirin), anticonvulsants, and corticosteroids—is the antipsychotics.
Antipsychotics have traditionally been used to treat disorders such as schizophrenia, in addition to treatment-resistant depression. Recent data, however, have shown that antipsychotics may play a role in chronic pain management. Current pain-management guidelines, such as those from the CDC and the Institute for Clinical Systems Improvement, have not addressed the use of this medication class for analgesia.6,7 There is some evidence from human and animal studies that suggests that the atypical antipsychotics may be an option for the adjunctive treatment of certain pain disorders, including migraine headaches, chronic pain, neuropathic pain, and fibromyalgia.
PATHOPHYSIOLOGY
Pain is a subjective sensation described as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” by the International Association for the Study of Pain.8 The sensation of pain generally begins with a nociceptive input from either actual or possible tissue injury, sending signals up the afferent pathway to the dorsal horn of the spinal cord. Neurotransmitters are then released that activate pathways reaching the brain, resulting in the perception of pain.9 Neuropathic pain is pain caused by a lesion or disease of the nervous system. Pain is classified as acute when it lasts less than 3 months and chronic when it lasts longer than 3 months. Pain can be either nociceptive or neuropathic and is further categorized as mild, moderate, or severe.
How pain is individually perceived is often influenced by cultural, spiritual, and societal factors. Depending on the individual’s personal pain-tolerance threshold, the presence of pain can range from an unpleasant nuisance to an extremely debilitating condition. Thus, it is important not only to treat pain but to do it in an appropriate manner that improves quality of life while minimizing the effects of physiological and psychological dependence.6,8,9
ANTIPSYCHOTICS’ MECHANISM OF ACTION
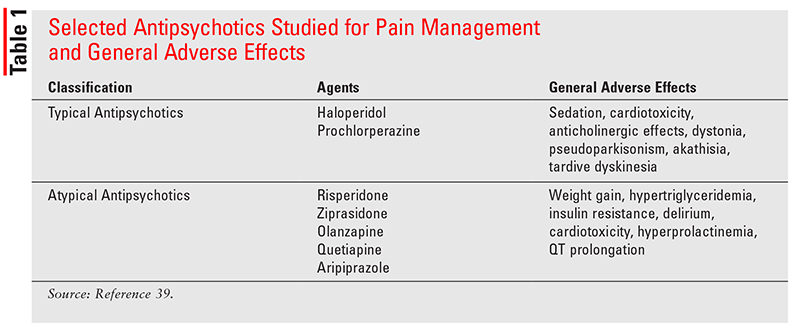
Antipsychotics are divided into two categories: first-generation, or typical antipsychotics, and second-generation, or atypical antipsychotics. The typical antipsychotics were first marketed in the 1950s for psychosis, but their use was limited by the risk of QT prolongation, sedation, and anticholinergic side effects, as well as severe extrapyramidal side effects (EPS) including acute dystonic reaction, pseudoparkinsonism, akathisia, and tardive dyskinesia. The atypical antipsychotics were first introduced around the 1990s and were associated with less severe neuromuscular side effects than the first generation. Unfortunately, the atypicals are more likely to cause serious metabolic effects such as weight gain, hypertriglyceridemia, hyperglycemia, and low-density lipoprotein levels.10
The principal difference between the two categories of antipsychotics results from their primary mechanism of action and relative selectivity in the brain. First-generation antipsychotics have the ability to block postsynaptic dopamine D2 receptors in the dopaminergic pathways of the brain. The binding is not entirely selective, resulting in the antagonism of dopamine receptors in the mesolimbic, mesocortical, tuberoinfundibular, and nigrostriatal pathways. This nonselectivity results in the undesirable and sometimes severe side effects. Typical antipsychotics also have noradrenergic, cholinergic, and histaminergic blocking action. The second-generation antipsychotics also block post-synaptic dopamine D2 receptors but primarily bind 5-HT2A serotonin receptors, offering a less dopamine-based binding profile than the typical antipsychotics, resulting in an overall reduced risk of EPS.11 Additionally, some antipsychotics such as aripiprazole and cariprazine have partial dopamine-receptor agonism.11
The antipsychotic class remains one of the most complex in all of psychopharmacology, given their distinct receptor-binding profiles. The mechanism by which antipsychotics achieve this effect has not yet been fully elucidated. Limited studies have been conducted in this area, and many experts are calling for further research to be done. The primary questions concern whether antipsychotics have analgesic properties and where in the body the drugs exert their pharmacologic effects.
Schreiber and colleagues hypothesized that the major target of antinociceptive action is in the midbrain of the central nervous system, through the connections of the dorsal raphe nucleus, which is rich in serotonin, and the periaqueductal gray region, which is rich in opioid receptors and endogenous opioids.12 A study in 2015 performed on mouse models by Kim and colleagues demonstrated an effect of dopaminergic neurons in maintaining pathological pain plasticity. The researchers studied three descending modulatory systems: serotonin, norepinephrine, and dopamine. The study found that serotonin is required for hyperalgesic priming initiation and norepinephrine for priming maintenance. Dopamine was found to play a role in both initiation and maintenance of hyperalgesic priming; more specifically, this mechanism was D1/D5 dependent.13 Schreiber and colleagues also found that analgesic mechanisms differed among the atypical antipsychotics themselves.12 Another postulated mechanism is through the atypical antipsychotic binding of the 5-HT2A receptor.14
Typical antipsychotic agents have been postulated to offer analgesia as well, but multiple studies have failed to demonstrate their efficacy in chronic pain management. This lack of efficacy in pain syndromes may be attributed to their lack of effects on neurotransmitter activity associated with the pain pathways.15 Because of their lesser tendency to cause EPS and other adverse effects (Table 1), atypical antipsychotics are more likely to be studied and tried for their analgesic use. Atypical antipsychotics, such as olanzapine, have also been studied to alleviate the pain of cluster headaches. Olanzapine has antagonistic properties at dopamine D1-4 receptors, serotonin 5-HT2A/2C receptors, muscarinic M1-5 receptors, histamine H1 receptors, and alpha-adrenergic receptors. Pain relief from cluster headaches may be due to olanzapine’s dopamine-receptor blockade or potential alpha-adrenergic antagonism activity, especially since dihydroergotamine, another cluster abortive agent, also has alpha-adrenergic receptor properties. Additionally, animal models have demonstrated enhanced analgesia with dopamine 1-2 receptor agonist activity.16 The histamine, serotonin, and muscarinic antagonist pathways are not likely to play a role in the mechanism of alleviation for cluster headaches.17 Other somatic syndromes, such as fibromyalgia, have also been postulated to work through the same pathways.
THERAPEUTIC APPROACHES
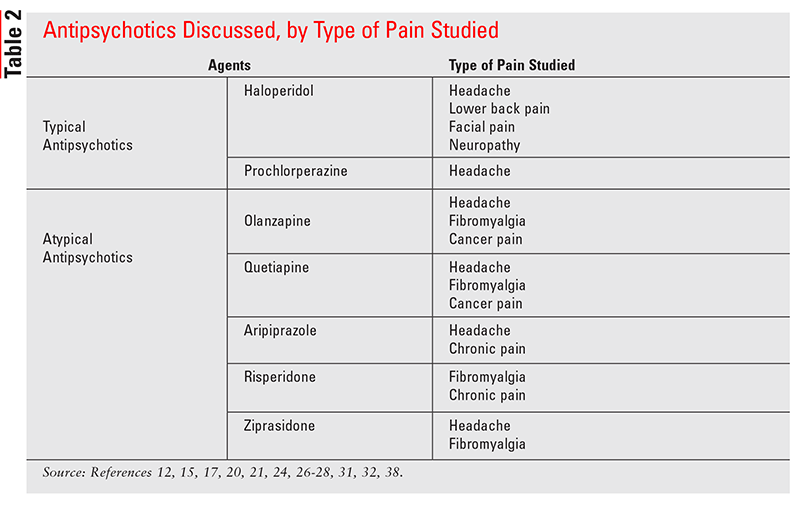
The most commonly prescribed antipsychotics with published studies in pain management include olanzapine, quetiapine, risperidone, aripiprazole, and ziprasidone (Table 2). Olanzapine and quetiapine have the most studies among the preceding five agents. Olanzapine, however, was also the only agent with robust clinical trial designs that showed consistent efficacy in fibromyalgia and headache/migraine- related pain.15
Headaches
Headache disorders are considered one of the most common disorders of the nervous system, categorized by migraines, cluster headache, and tension headaches. The World Health Organization estimates that nearly 50% of adults worldwide have some sort of headache disorder, with higher incidence in women than men.18 Treatment encompasses a wide variety of nonpharmacologic and pharmacologic measures. Simple analgesics, steroids, opioids, anticonvulsants, antihypertensives, triptans, or ergot derivatives can be used in the management of headache pain.
Olanzapine is the most frequently cited agent in the literature for treatment of headaches and migraines, and it has been assessed by five studies; four of the five demonstrated efficacy.15 One randomized, controlled trial (RCT) included patients admitted to the emergency department as a result of a primary headache. The patients were given IM olanzapine for treatment. In the olanzapine group, 38 of 44 (86.4%) patients who had reported moderate or severe pain at baseline reported mild or no pain at 60 minutes.19 Two case series also involved quetiapine and aripiprazole for headaches, both of which were effective. In both episodic and chronic cluster headaches, olanzapine was shown to reduce pain by up to 80% in four of the five patients studied, with two patients having their headache completely resolved.17
Typical antipsychotics have also been studied for headache relief. Acute migraine, chronic tension headache, and headache after spinal tap trials studied showed statistically significant results with haloperidol.20 A retrospective analysis of inpatients suffering from refractory chronic headaches on a daily basis who received repeated doses of prochlorperazine IV found that after treatment, patients felt a 50% or greater reduction in their headache pain, with 63% of them achieving headache resolution.21
Chronic Pain
Lower back pain is common and may affect patients of all ages, but peak prevalence occurs between ages 35 and 55.22 Chronic lower back pain is pain that persists for more than 12 weeks despite the injury or underlying cause having been addressed.20 Nearly 80% of adults experience back pain at some point in their lives, with equal incidence between men and women.23 As early as 1976, a German article reported benefits with the use of haloperidol for lower back pain.24
Another type of chronic pain includes facial pain, which is a condition that can occur as a result of headaches or injuries. Trigeminal neuralgia is a type of facial pain caused by a disorder of the 5th cranial nerve (the trigeminal nerve) and is characterized by spontaneous shock-like pain in areas of the face where the trigeminal nerves exists. The pain can last from seconds to minutes. Pharmacologic treatment includes anticonvulsants.25 As early as 1978, there have been case reports of patients whose facial pain was ameliorated with antipsychotics. Raft and colleagues reported 12 patients with refractory chronic facial pain that Improved after behavioral relaxation therapy and adjunctive haloperidol.26
With regard to generalized chronic pain, olanzapine has been described in three case studies as having been successful in regulating pain perception in adults.27 Aripiprazole also showed efficacy in four reported cases of patients with varying sources for their chronic pain.28
Cancer Pain
Cancer pain is often chronic and may be due to tumor, cancer treatment, or unrelated etiologies. It may be nociceptive, which is due to physical injury, or neuropathic. Advanced cancer pain is frequently characterized by chronic continuous pain with intermittent acute breakthrough pain. Up to 59% of cancer patients reported pain while undergoing cancer treatment, with 64% of patients reporting advanced cancer pain. In addition, up to 33% of patients were still reporting pain after curative treatment.29 Pharmacotherapy is the mainstay of cancer-pain treatment, with opioids playing a large role in managing the debilitating pain. In addition to pharmacologic treatment, psychosocial and spiritual care may be necessary to help alleviate pain. The WHO cancer pain-management guidelines recommend nonopioid analgesics as appropriate at the initiation of therapy based on the patients’ reported pain score, with escalation to opioid therapy as warranted.30 The National Comprehensive Cancer Network guidelines offer a similar suggestion.29
There are not many studies available regarding the use of antipsychotics for cancer pain. A study performed on mouse models in Korea examined the use of quetiapine on cancer-induced bone pain; it suggested that quetiapine may have analgesic effects through reduction of certain receptor targets in cancer-induced bone pain.31 In another study of eight cancer patients with severe, uncontrolled pain despite opioid titration, olanzapine was found to have caused a noticeable reduction in their daily pain scores, and average daily opioid use decreased significantly in all patients. These patients had a nonspecific cognitive disorder, and their cognitive impairment and anxiety also appeared to be reduced within a day after treatment with olanzapine. The authors postulated that olanzapine’s analgesic effect may be due to either the improvement in cognitive performance or an intrinsic analgesic mechanism.32
Fibromyalgia
Fibromyalgia is a multifaceted disorder characterized by extensive chronic, generalized pain associated with various somatic symptoms including stiffness, balance problems, and hypersensitivity to physical and psychological environmental stimuli.33 Musculoskeletal pain can last more than 3 months with concomitant fatigue and mood changes, as well as sleep and cognitive disturbances. It is estimated that nearly 4 million Americans have fibromyalgia.34 Management is multidisciplinary, with nonpharmacologic and pharmacologic measures. Pharmacologic treatment consists of serotonin-norepinephrine reuptake inhibitors, pregabalin, gabapentin, and anticonvulsants.35 Common analgesics are usually not effective for this syndrome. The number of patients who are able to attain notable pain relief falls short, with efficacy only 10% to 25% greater than placebo.36
In general, the largest amount of research for support of antipsychotic analgesia is for fibromyalgia-related pain. Quetiapine, olanzapine, and ziprasidone have the most research for this disorder. To date, there have been a total of seven studies examining antipsychotic analgesia in fibromyalgia: three studies assessing olanzapine, three studies assessing quetiapine, and one study assessing ziprasidone. Olanzapine was the only agent for which studies were able to consistently demonstrate efficacy in fibromyalgia, showing benefits in pain levels, overall activity levels, and decreased interference with mood, work, and sleep.15
Neuropathic Pain
Neuropathic pain is characterized by hypersensitivity to stimuli and a pain response to stimuli that does not normally cause pain. It is estimated to have a prevalence of 3% to 17% in the general population.37 Current pharmacologic treatment consists of anticonvulsants, antidepressants, or topical agents. Neuropathic pain may be difficult to treat and is often treatment-resistant, therefore supporting the need for alternative agents. The sigma-1 receptor is known to be activated during neuropathic pain and may serve as a potential pharmacotherapy target. A study performed by Espinosa-Juarez and colleagues aimed to find analgesic benefit with haloperidol, which has been thought to antagonize sigma-1.38 The researchers compared haloperidol to a sigma-1 antagonist control and gabapentin as the positive control in rats with chronic constriction injury. They discovered that haloperidol held the same efficacy as the other two agents, while exhibiting higher potency. Overall, their results suggested that haloperidol exerts its antinociceptive effects at the spinal level through sigma-1 blockade.38 More research is needed, however, to assess its role in humans with neuropathic pain.
CONCLUSION
Pharmacists are in a key position to help patients obtain the pain treatment they need while preventing opioid misuse. Given the addiction, dependence, tolerance, and overdose potential of opioid analgesics, pharmacists must be mindful of other treatment options and be prepared to educate patients on their efficacy, monitoring parameters, and safety concerns.
There is limited evidence for the use of antipsychotics in the management of various types of pain. Limitations include potential adverse effects, safety concerns, and extensive monitoring parameters. In addition, more double-blind, randomized, placebo-controlled studies are needed to fully assess the role of antipsychotics in pain management. Although literature is limited and there are no current pain-management guidelines recommending the use of antipsychotics as a treatment option, there may be a stronger push for this class of medications to be used off-label as physicians look for safer alternatives to opioid analgesics. All providers who are part of the interdisciplinary team should be aware of the potential role of antipsychotics. Community pharmacists who perform final verifications on medications should now keep in mind this potential alternative use for antipsychotics as an upcoming tool to help tackle chronic pain and, it is hoped, provide relief for patients.
REFERENCES
1. Dipiro JT, Talbert R, Yee G, et al. Pharmacotherapy: A Pathophysiologic Approach, 10th ed. New York: McGraw-Hill Medical, 2017.
2. United States Census Bureau. www.census.gov/quickfacts/fact/table/US/PST045218. Accessed on December 19, 2019.
3. Bernard SA, Chelminski PR, Ives TJ, et al. Management of pain in the United States—a brief history and implications for the opioid epidemic. Health Serv Insights. 2018;11:1-6.
4. CDC. Opioid overdose: prescribing data. www.cdc.gov/drugoverdose/data/prescribing.html. Accessed on December 5, 2019.
5. Substance Abuse and Mental Health Services Administration. (2019). Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. www.samhsa.gov/data/. Accessed on December 19, 2019.
6. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
7. Institute for Clinical Systems Improvement. Pain: assessment, non-opioid treatment approaches and opioid management. August 2017. Eighth Edition, Version 2. www.icsi.org/. Accessed February 29, 2020
8. International Association for the Study of Chronic Pain. IASP terminology. Pain. www.iasppain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576#Pain. Accessed February 19, 2020.
9. Vanderah TW. Pathophysiology of pain. Med Clin North Am. 2007; 91(1):1-12.
10. Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry 2004;6(Suppl2): 8-13.
11. Metzler HY. What’s atypical about atypical antipsychotic drugs? Curr Opin Pharmacol. 2004;4:53-57.
12. Schreiber S, Getslev V, Backer MM, et al. The atypical neuroleptics clozapine and olanzapine differ regarding their antinociceptive mechanisms and potency. Pharmacol Biochem Behav. 1999;64(1):75-80.
13. Kim JV, Tillu DV, Quinn TL, et al. Spinal dopaminergic projections control the transition to pathological pain plasticity via a d1/D5-mediated mechanism. J Neurosci. 2015;35(16):6307-6317.
14. Sutherland AM, Nicholls J, Bao J, et al. Overlaps in pharmacology for the treatment of chronic pain and mental health disorders. Prog in Neuropsychopharmacol BiolPsychiatry. 2018;87(Pt B):290-297.
15. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management. Clinc J Pain. 2018;34(6):585-591.
16. Wang HH, Xu SF. Effect of D1 and D2 dopamine receptor antagonists on acupuncture analgesia. Sheng Li Xue Bao. 1993;45:61-68.
17. Rozen TD. Olanzapine as an abortive agent for cluster headache. Headache. 2001;41:813-816.
18. World Health Organization. Headache disorders. Who.int/news-room/fact-sheets/detail/headache-disorders. Accessed December 4, 2019.
19. Hill CH, Miner JR, Martel ML. Olanzapine versus droperidol for the treatment of primary headache in the emergency department. Acad Emerg Med. 2008;15(9):806-811.
20. Seidel S, Aigner M, Ossege M, et al. Antipsychotics for acute and chronic pain in adults. Cochrane Database Syst Rev. 2013;8:CD004844.
21. Lu SR, Fuh JL, Juang KD, et al. Repetitive intravenous prochloperazine treatment of patients with refractory chronic daily headache. Headache. 2000;40:724-729.
22. World Health Organization. Background paper 6.24 Low back pain. Who.int/medicines/areas/priority_medicines/BP6_24LBP.pdf. Accessed December 4, 2019.
23. National Institutes of Health. National Institute of Neurological Disorders and Stroke. Low back pain fact sheet. Ninds.nih.gov/disorders/patient-caregiver-education/fact-sheets/low-back-pain-fact-sheet. Accessed December 6, 2019.
24. Kocher R. The treatment of chronic pain symptoms with psychotropic drugs. Pharmakopsychiatr Neuropsychopharmakol. 1976;9(6):337-341.
25. Facial Pain Association. What is Trigeminal Neuralgia? Fpa-support.org/learn/. Accessed December 8, 2019.
26. Raft D, Toomey T, Gregg JM. Behavior modification and haloperidol in chronic facial pain. South Med J. 1979;72(2):155-159.
27. Gorski ED, Willis KC. Report of three case studies with olanzapine for chronic pain. J Pain. 2003;4(3):166-168.
28. Kasahara S, Kunii Y, Mashiko H, et al. Four cases of chronic pain that improved dramatically following low-dose aripiprazole administration. Prim Care Companion CNS Disord. 2011;13(2).
29. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. JNCCN. 2019;17(8):1-31.
30. World Health Organization. WHO Guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents. January 2019. www.who.int/ncds/management/palliative-care/cancer-pain-guidelines/en/. Accessed February 20, 2020.
31. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32:1069-1074.
32. Khojainova N, Santiago-Palma J, Kornick C, et al. Olanzapine in the management of cancer pain. J Pain Symptom Manage. 2002;23(4):346-350.
33. Calandre EP, Rico-Villademoros F. The role of antipsychotics in the management of fibromyalgia. CNS Drugs. 2012;26:135-153.
34. CDC. Fibromyalgia. cdc.gov/arthritis/basics/fibromyalgia.htm. Accessed December 12, 2019.
35. Kwiatek R. Treatment of fibromyalgia. Aust Presc. 2017;40(5):179-183.
36. Walitt B, Klose P, Üçeyler N, et al. Antipsychotics for fibromyalgia in adults. Cochrane Database Syst Rev. 2016;6:CD011804.
37. Cavalli E, Mammana S, Nicoletti F, et al. The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol. 2019;33.
38. Espinosa-Juarez JV, Jaramillo-Morales OA, Lopez-Munoz FJ. Haloperidol decreases hyperalgesia and allodynia induced by chronic constriction injury. Basic Clin Pharmacol Toxicol. 2017;121:471-479.
39. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1)3-11.
To comment on this article, contact rdavidson@uspharmacist.com.






