Alzheimer’s disease (AD) is a degenerative brain disease and the main cause of dementia. Dementia is a neurodegenerative disease characterized by the gradual loss of memory, speech, judgment, reasoning, and other mental abilities. Dementia also affects a person’s ability to perform everyday activities.1
In AD, the damage and degeneration of the brain cells eventually affect other parts of the brain, including those that help a person to perform basic bodily functions such as walking, swallowing, and talking. People with AD are bed-bound during the final stages of the disease and require full attention and care. AD is eventually a fatal disease.1
AD is officially listed as the sixth leading cause of death in America. It is also the fifth leading cause of death for those aged >65 years. Similarly, AD is a leading cause of disability and morbidity. Patients with AD live through years of morbidity and poor health before death.1
There are currently 5.4 million Americans suffering with AD. Around the world, the number is estimated to be as high as 35 million. It is projected that if a breakthrough discovery is not found to treat AD, the number of patients with AD worldwide can be as high as 60 million by 2030.1
While there is no cure for AD, there are five prescription drugs approved by the FDA to treat its symptoms.
ETIOLOGY
Alzheimer’s disease was first identified more than 100 years ago by Alois Alzheimer, a German physician. Before it was recognized as the most common cause of dementia as well as a major cause of death, 70 years passed. AD accounts for an estimated 60% to 80% of dementia cases. Difficulty remembering recent events, conversations, or names is often an early clinical symptom. Lack of interest and depression are also often early symptoms.2
Revised criteria and guidelines for diagnosing AD were proposed and published in 2011. They recommend that AD be considered a slowly progressive brain disease that begins well before clinical symptoms emerge.
AD progresses through three stages. These are mild (early stage), moderate (middle stage), and severe (late stage). Although the symptoms get worse from the early to the late stage, it may be difficult to place a person with AD in a specific stage.3
The main pathologies of AD are the progressive accumulation of the protein fragment beta-amyloid outside neurons in the brain and twisted strands of the protein tau inside neurons. These changes are eventually accompanied by damage to and death of neurons.
Researchers believe that early detection of AD will be key to preventing, slowing, and stopping the disease.2
Other diseases that cause dementia are Parkinson’s disease (PD), Huntington disease (HD), Creutzfeldt-Jakob disease (CJ), vascular dementia, Lewy body dementia, poststroke dementia (<10%), and hydrocephalus (<5%).3
SIGNS AND SYMPTOMS
The speed at which symptoms advance from mild to moderate to severe varies from person to person. As AD progresses, cognitive and physical abilities decline. In the more advanced stages, people need help with basic activities of daily living as they lose their ability to communicate, fail to recognize loved ones, and become bed-bound and need around-the-clock care. When individuals have difficulty moving, they are more vulnerable to infections, including pneumonia.4
The following signs and symptoms may be observed in persons with AD: memory loss that disrupts daily activities; difficulty in planning or in solving problems; struggling to complete familiar tasks at home and work; disorientation with time or place; trouble understanding visual images and spatial objects; difficulty with words when speaking or writing; misplacing things and losing the ability to find them; poor judgment; absence in the workplace or at social activities and increased anxiety; agitation and depression; and sleep disturbances.5
DIAGNOSIS
A careful and comprehensive medical evaluation is required for diagnosing AD. Although physicians (neurologists) can almost always determine if a person has dementia, it may be difficult to identify the exact cause. Several days or weeks may be needed for the patient to complete the required tests and examinations and for the physician to interpret the results and make a diagnosis.6
A healthy adult brain has about 100 billion neurons, each with long, branching extensions. These extensions enable individual neurons to form connections with other neurons. At these connections, called synapses, information flows in tiny bursts of chemicals (e.g., acetylcholine) that are released by one neuron and detected by a receiving neuron. The brain contains about 100 trillion synapses. They allow signals to travel rapidly through the brain’s neuronal circuits, creating the cellular basis of memories, thoughts, sensations, emotions, movements, and skills.6
The accumulation of the protein beta-amyloid (called beta-amyloid plaques) outside neurons and the accumulation of an abnormal form of the protein tau (called tau tangles) inside neurons are two of several brain changes believed to contribute to the damage and destruction of neurons that result in memory loss and other symptoms of AD. As brain changes advance, information transfer at synapses begins to fail, the number of synapses declines, and neurons eventually die.
The accumulation of beta-amyloid is believed to interfere with the neuron-to-neuron communication at synapses and to contribute to cell death. Tau tangles block the transport of nutrients and other essential molecules inside neurons and are also believed to contribute to cell death. The brains of people with advanced AD show inflammation, dramatic shrinkage from cell loss, and widespread debris from dead and dying neurons.6
Today, brain imaging, neuroimaging, and benefits from the biomarkers that are sensitive to brain changes are among the most promising areas of research to find a cure for AD.
APPROVED MEDICATIONS AND EFFICACY
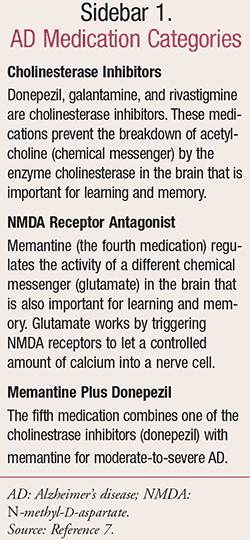
There are five drugs approved by the FDA for AD, and they are divided into the three categories shown in SIDEBAR 1. Currently, these are available treatment options that can help persons with AD to improve their symptoms and quality of life.7
Mode of Action
Once the message passes the synapse and reaches the receiving cell, an enzyme, acetylcholinesterase, breaks acetylcholine down so it can be recycled. AD damages or destroys cells that produce and use acetylcholine, thereby reducing the amount available to carry messages. Cholinesterase inhibitors block acetylcholinesterase and increase acetylcholine levels, thus helping deliver messages to other cells. Cholinesterase inhibitors also appear to stimulate the release of acetylcholine and strengthen its activity (galantamine). Rivastigmine may block the activity of another enzyme that is involved in breaking down acetylcholine.7,8
Cholinesterase inhibitors cannot reverse AD and cannot stop the destruction of nerve cells. As a result, their ability to improve AD symptoms gradually declines as brain cells die. The effectiveness of acetylcholinesterase inhibitors as well as how long they are effective varies from person to person. There is evidence that people with moderate-to-severe AD can benefit more if they take acetylcholine inhibitors with memantine.8
Memantine is a glutamate-pathway modifier. It may regulate the abnormal activity of glutamate, which is another chemical messenger in the brain important for memory and learning. Glutamate is a powerful excitatory neurotransmitter released by nerve cells in the brain. It is also responsible for sending signals between the brain cells. However, at high concentrations, the increased cellular activity caused by glutamate results in overexcitation of nerve cells by allowing too much calcium into the nerve cells, which eventually leads to cell death. Memantine shields brain cells from overexposure to glutamate. It is not yet proven that this drug reverses AD symptoms in the long-term.8
Donepezil
This drug is a cholinesterase inhibitor, and it is used in all stages of Alzheimer’s dementia. It is available in 5-, 10-, and 23-mg tablets and approved to be used for mild-to-severe forms of AD.9
Mild-to-Moderate: Initially 5-mg once daily; may increase to 10 mg once daily after 4 to 6 weeks; effective dosage range in clinical studies: 5 to 10 mg/day.
Moderate-to-Severe: Initially 5 mg once daily; may increase to 10 mg once daily after 4 to 6 weeks; may increase further to 23 mg once daily after ≥3 months.
Off-label Uses: Dementia associated with Parkinson’s disease: 5 mg once daily; may increase to 10 mg once daily after 4 to 6 weeks; may increase further to 23 mg once daily after the use of 10 mg once daily ≥3 months. Lewy body dementia: Initially 3 mg once daily for 2 weeks, then increase to 5 mg once daily. After 4 weeks may further increase dose based on response and tolerability up to 10 mg once daily. There is no need for dose adjustment in renal or hepatic impairment. The drug can be administered at bedtime without regard to food.9
Donepezil 23-mg Tablet: Do NOT crush or chew owing to an increased rate of absorption. The 23-mg strength is provided in a unique film-coated formulation different from the 5- or 10-mg tablet strengths, which results in an altered pharmacokinetic profile.
Donepezil ODT: Allow orally disintegrating tablet to dissolve completely on tongue and follow with water.
Side Effects: Patient may experience diarrhea, insomnia, loss of strength and energy, muscle cramps, lack of appetite, weight loss, or headache. Patients should report severe dizziness, bradycardia, arrhythmia, difficult urination, seizures, heartburn, severe abdominal pain, and black, tarry, or bloody stools immediately to the prescriber.10
Galantamine
This drug is a cholinesterase inhibitor and is used in mild-to-moderate AD. Immediate-release (IR) tablet or solution: Initially 4 mg twice daily for 4 weeks; if tolerated, increase to 8 mg twice daily for ≥4 weeks; if tolerated, increase to 12 mg twice daily.9
Extended-release (ER) Capsule: Initially 8 mg once daily for 4 weeks; if tolerated, increase to 16 mg once daily for ≥4 weeks; if tolerated, increase to 24 mg once daily. If therapy is interrupted for ≥3 days, restart at the lowest dose and increase to current dose.
Severe AD (Off-label Use): IR tablet: 4 mg twice daily for 4 weeks; if tolerated, increase to 8 mg twice daily for ≥4 weeks; if tolerated, increase to 12 mg twice daily. May decrease to 8 mg twice daily if the target dose is not tolerated.
Dementia Associated With Parkinson’s Disease and Lewy Body Dementia (Off-label Use): American Psychiatric Association recommends dosing and titration similar to those for patients with AD.9,10
Conversion From IR to ER Formulation: Patients may be switched from the IR formulation to the ER formulation by taking the last IR dose in the evening and beginning the ER dose the following morning; the same total daily dose should be used.
Conversion to Galantamine From Other Cholinesterase Inhibitors: Patients experiencing poor tolerability with donepezil or rivastigmine should wait until side effects subside or allow a 7-day washout period prior to beginning galantamine. Patients not experiencing side effects with donepezil or rivastigmine may begin galantamine therapy the day immediately following discontinuation of previous therapy. In geriatric patients with low body weight and/or serious comorbidities, adjust dose with caution.9
There are no dosage adjustments provided in the manufacturer’s labeling for mild renal impairment. Moderate impairment (creatinine clearance [CrCl] 9-59 mL/min): Maximum dose is 16 mg daily. Use is not recommended in severe impairment (CrCl <9 mL/min).
In mild hepatic impairment (Child-Pugh score 5-6), there are no dosage adjustments provided in the manufacturer’s labeling. In moderate impairment (Child-Pugh score 7-9), the maximum dose is 16 mg daily. Use is not recommended in severe impairment (Child-Pugh score 10-15).10
Rivastigmine
This medication is a cholinesterase inhibitor and is used in mild-to-moderate AD. The initial dose is 1.5 mg twice daily; may increase by 3 mg daily (1.5 mg/dose) every 2 weeks based on tolerability (maximum dose, 6 mg twice daily). Careful titration and monitoring should be performed in patients with low body weight. In patients <50 kg, monitor closely for toxicities (e.g., excessive nausea, vomiting) and consider reducing the dose if such toxicities develop.9
If gastrointestinal (GI) adverse events occur, discontinue treatment for several doses, then restart at the same or next lower dosage level; antiemetics have been used to control GI symptoms. If dosing is interrupted for ≥3 days, restart the treatment at the same or lower dose and titrate as previously described.
Transdermal Patch: Apply 4.6 mg/24 hours patch once daily; if well tolerated, may titrate to 9.5 mg/24 hours (continue as long as therapeutically beneficial) and then to 13.3 mg/24 hours (maximum dose).10
If dosing is interrupted for ≥3 days, restart treatment with the same or a lower strength patch. If interrupted for >3 days, reinitiate at 4.6 mg/24 hours and titrate to lowest effective maintenance dose. If oral daily dose <6 mg, switch to 4.6 mg/24 hours patch; if oral daily dose 6-12 mg, switch to 9.5 mg/24 hours patch. Apply patch on the day following last oral dose.10
Severe AD: Initially apply 4.6 mg/24 hour patch once daily. Titrate dose as recommended for transdermal dosing for mild-to-moderate AD.
In mild-to-moderate Parkinson-related dementia, use 1.5 mg twice daily; may increase by 3 mg daily every 4 weeks based on tolerability (maximum dose, 6 mg twice daily).
Cholinesterase inhibitors are recommended for treatment of neuropsychiatric symptoms associated with Lewy body dementia; however, rivastigmine is the only cholinesterase inhibitor with placebo-controlled data supporting its use. In patients who do not respond to cholinesterase inhibitors, a cautious trial of an atypical antipsychotic agent may be warranted after warning the patient and caregiver about the potential for severe sensitivity reactions.10
Memantine
This drug is an N-methyl-D-aspartate (NMDA) receptor antagonist, and it is used in treating moderate-to-severe AD.9
IR: Start with 5 mg daily; increase dose by 5 mg daily to a target dose of 20 mg daily; wait ≥1 week between dosage changes. Doses >5 mg daily should be given in two divided doses. If treatment is interrupted for more than several days, the treatment may need to be restarted at a lower dose and retitrated.10
ER: Start with 7 mg once daily, increase dose by 7 mg daily to a target maximum dose of 28 mg once daily; wait ≥1 week between dosage changes (if previous dose well tolerated).
When switching from the IR product to the ER product, begin the ER product the day after the last dose of the IR product. Patients on IR 10 mg twice daily should be switched to ER 28 mg once daily. If a single dose is missed, patients should not double up on the next dose; take the next dose as scheduled. If several days of dosing are missed, dosing may need to be resumed at lower doses and retitrated.
In severe renal problems, when CrCl 5-29 mL/min, use the ER with caution. Start with 5 mg once daily; after at least 1 week of therapy and if tolerated, may titrate up to a target dose of 5 mg twice daily.10
Memantine Plus Donepezil
This medication is for moderate-to-severe AD. For patients stabilized on memantine (10 mg twice daily or 28 mg ER once daily) and donepezil 10 mg: Combination therapy should be started the day after the last dose of memantine and donepezil administered separately.
Mild-to-Moderate Renal Impairment (CrCl 30-80 mL/min): No dosage adjustment is necessary.
Severe Renal Impairment (CrCl 5-29 mL/min): Patients stabilized on memantine (5 mg twice daily or 14 mg ER once daily) and donepezil 10 mg may be switched to memantine ER 14 mg/donepezil 10 mg once daily.9
Mild-to-Moderate to Severe Hepatic Impairment (Child-Pugh Class C): There are no dosage adjustments provided in the manufacturer’s labeling (has not been studied).10
Administration: Administer in the evening without regard to meals. Patients should swallow capsule whole and not divide, chew, or crush. May open capsule and sprinkle entire contents on applesauce and swallow immediately.10
CONCLUSION
AD is a degenerative brain disease and the main cause of dementia. There are currently 5.4 million Americans living with AD. It is now believed that the accumulation of the protein beta-amyloid outside neurons and an abnormal form of the protein tau inside neurons result in memory loss and other symptoms. While there is no cure, there are currently several available treatment options for patients with AD that can improve their symptoms and quality of life. Worldwide medical research is under way for a breakthrough discovery to cure this destructive disease.
REFERENCES
1. Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11:332-384.
2. National Institute on Aging. About Alzheimer’s disease: mild cognitive impairment. www.nia.nih.gov/alzheimers/topics/mild-cognitive-impairment. Accessed June 15, 2016.
3. National Center for Health Statistics (2010). Alzheimer’s disease. www.cdc.gov/nchs/fastats/alzheimr.htm. Accessed July 15, 2016.
4. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. AlzheimersDement. 2011;7(3):280-292.
5. Marder K. Dementia and memory loss. In JC Brust, ed. Current Diagnosis and Treatment Neurology. 2nd ed. New York, NY: McGraw-Hill; 2012:78-101.
6. McKhann GM, Knopmanc DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
7. Small SA, Mayeux R. Alzheimer disease. In: Rowland LP, Pedley TA, eds. Merritt’s Neurology. 12th ed. Philadelphia, PA: Lippincott Williams and Wilkins:; 2010:713-718.
8. Baumgart M, Snyder HM, Carrillo MC, et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11:718-726.
9. Qaseem A, Vincenza S, Cross T Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008;148:370-378.
10. Lexicomp online. www.wolterskluwercdi.com/lexicomp-online Accessed July 2016.





