US Pharm. 2007;32(9):74-76.
Diabetes is the fifth leading
cause of death worldwide.1 It is characterized by increased levels
of blood sugar resulting from defects in insulin secretion, insulin
sensitivity, or both. There are two types of diabetes; type 1 diabetes results
from an autoimmune disorder that causes beta-cell destruction, while type 2
diabetes results from both a progressive defect in insulin secretion and
insulin sensitivity to receptors.2 Research has demonstrated that
improved glycemic control is associated with sustained, decreased rates of
microvascular (i.e., retinopathy and nephropathy), macrovascular (i.e.,
myocardial infarction and stroke), and neuropathic complications.
Cardiovascular disease, a
major cause of death in the United States, kills nearly 500,000 Americans each
year.3 The principle causative factor, hyperlipidemia, is due to an
elevation of one or more of the following: cholesterol, phospholipids, or
triglycerides circulating in the plasma. Uncontrolled levels of lipids can
increase the risk of premature death.4
Monitoring
Physical activity,
weight loss, and proper diet can help maintain normal levels of glucose and
cholesterol. One very important method of tracking the progress of diabetes is
to monitor the patient's glucose on a regular basis.5
Fortunately, there are many devices that allow such readings in the
convenience of one's home. However, testing for cholesterol levels is almost
exclusively performed in the physician's office by drawing blood and sending
the sample to a lab at another facility, making cholesterol testing difficult
to obtain for some patients. Fortunately, the Q.STEPS Biometer G/C Dual
Monitoring System (Q.STEPS) is a home diagnostic device that checks both
glucose and cholesterol levels, affording patients' convenience and accuracy.
Major clinical trials
assessing the impact of glycemic control on diabetes complications have
included self-monitoring of blood glucose (SMBG) as part of multifactorial
interventions, suggesting that SMBG is a component of effective therapy. SMBG
allows patients to evaluate their individual response to therapy and assess
whether glycemic targets are being achieved. The most common values measured
for diabetes are glucose levels and glycosated hemoglobin. The American
Diabetes Association's 2006 practice guidelines recommend the following
clinical targets for adults with diabetes: hemoglobin A1c below 7%, fasting
blood glucose at 90 to 130 mg/dL, and a postprandial plasma glucose level of
less than 180 mg/dL.2
Monitoring cholesterol is an
integral part of cholesterol management and commonly includes a fasting
lipoprotein profile. This profile measures total cholesterol, low-density
lipoprotein (LDL) cholesterol, triglyceride, and high-density lipoprotein
(HDL) levels in the plasma. The Adult Treatment Panel (ATP) III guidelines
recommend the following clinical targets for adults: total cholesterol level
of less than 200 mg/dL, LDL cholesterol level of less than 100 mg/dL,
triglyceride level of less than 150 mg/dL, and HDL cholesterol level of
greater than 60 mg/dL.6
Q.STEPS Biometer G/C Dual
Monitoring System
Q.STEPS is intended
for in vitro diagnostic use with whole human blood. The system consists of a
user's manual, a calibration chip, glucose test strips, cholesterol test
strips, a control solution, lancets, alcohol swabs, two AAA batteries, a quick
reference card, and a carrying case.
Before using the device to
check for blood glucose and/or cholesterol levels, a system check must be
performed with the control solution. This should be performed at least once
every three months, if the patient experiences symptoms that do not match the
results of the device, or when a new vial of test strips is opened.
Q.STEPS can accurately give
glucose and cholesterol readings between the ranges of 50 to 400 mg/dL and 150
to 350 mg/dL, respectfully, in less than 30 seconds. Excessive glucose and
cholesterol readings of 400 and 350 mg/dL, respectfully, are displayed as
"Hi," while those below 50 and 150 mg/dL, respectfully, are displayed as "Lo";
neither result will display a numerical value. The device contains a built-in
memory bank that is capable of storing the last 99 glucose readings and the
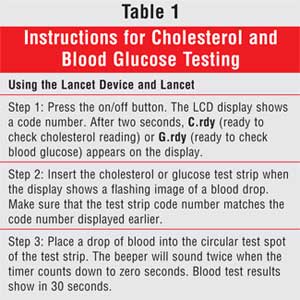
last 50 cholesterol readings. Patient instructions are detailed in Table 1
.
Limitations
Separate test
strips must be used to monitor glucose and cholesterol, and the two readings
cannot be performed simultaneously. Additionally, the meter does not indicate
whether the blood sample is adequate; an inaccurate glucose or cholesterol
reading may be obtained if the sample is not sufficient. In order to obtain
accurate results, approximately 3 and 15 mcL of blood should be obtained for
glucose and cholesterol testing, respectively.
Efficacy
Glucose:
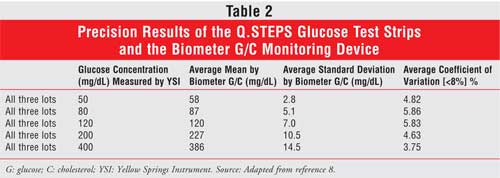
Precision results for Q.STEPS were determined following the National
Committee for Clinical Laboratory Standards (NCCLS) guidelines. The company
used glucose-spiked blood in concentrations of 50, 80, 120, 200, and 400
mg/dL. These samples were first measured by the Yellow Springs Instrument
(YSI), a standard biochemical analyzer, as a reference, and were subsequently
measured using Q.STEPS. Readings were taken twice per day for 20 days, and
each concentration level was applied to three lots of test strips. Precision
results were all within 6% and are presented in Table 2.
Method comparison studies for
linearity were performed at three different clinical sites. This correlation
study utilized finger-stick, whole-blood samples for comparison of the Q.STEPS
and YSI. It was shown that the glucose test results from Q.STEPS had a linear
relation of Y = 0.9976x + 1.8331 with a linear regression coefficient of R =
0.95, constituting compliance with universal guidelines.
Cholesterol:
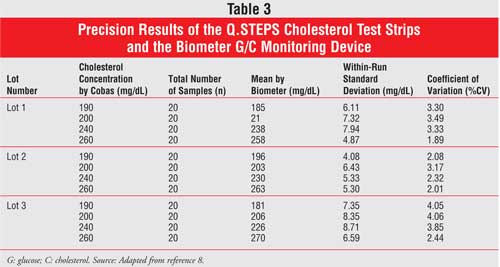
Precision results for the Q.STEPS were determined following NCCLS guidelines.
The company used cholesterol-spiked blood in concentrations of 190, 200, 240,
and 260 mg/dL. These samples were first measured by Cobas, as a reference, and
were then measured by Q.STEPS. Readings were taken twice per day, and each
concentration was applied to three lots of test strips. Precision results were
all within 6% and are presented in Table 3.
Method comparison studies for
linearity were compared with the Abel-Kendall reference method performed in a
CDC-certified Cholesterol Reference Method Laboratory Network (CRMLN).
External studies were also done at three clinical sites by lay-users. A total
of 456 patients participated in the clinical trial. It was shown that the
glucose results from Q.STEPS had a linear relation of Y = 0.9411x + 16.555,
with a linear regression coefficient of R = 0.986. The accepted standards for
cholesterol testing, CRMLN states that the regression analysis coefficient
variation should be r = 0.989. The regression analysis meets
cholesterol-monitoring requirements, constituting compliance with universal
guidelines.
Conclusion
Blood glucose
monitoring is a cornerstone in controlling diabetes, and blood cholesterol
monitoring has an important role in preventing cardiovascular disease in
patients. Q.STEPS provides patients with the convenience of having one machine
to monitor two different conditions in the comfort of their homes. Additional
information about Q.STEPS is available online at www.biomedixusa.com or via
telephone at (888) 246-6318.
References
1. Dream Trial
Investigators, Bosch J, Yusuf S, Gerstein HC, et al. Effect of ramipril on the
incidence of diabetes. N Engl J Med. 2006;355:1551-1562.
2. American Diabetes
Association. Standards of medical care in diabetes--2007. Diabetes Care
. 2007;30:S4-S41..
3. Centers for Disease
Control and Prevention Web site. Available at:
www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf. Accessed February 5, 2007.
4. Nissen S, Tuzcu EM,
Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and
coronary artery disease. N Engl J Med. 2005;352:29-38.
5. McMahon GT, Arky RA.
Inhaled insulin for diabetes mellitus. N Engl J Med. 2007;356:497-502.
6. Grundy SM, Cleeman
JI, Merz NB, et al. Implications of recent clinical trials for the national
cholesterol education program adult treatment panel III guidelines.
Circulation. 2004;110:227-239.
7. Biomedix Inc.
Q.STEPS Biometer G/C User's Manual. Fremont, CA, 2004.
8. U.S. Food and Drug
Administration. Available at: www.fda.gov. Accessed February 14, 2007.
To comment on this article, contact
editor@uspharmacist.com.





