US
Pharm. 2006;1:28-37.
"Once again, nature has
presented us with a daunting challenge: the possibility of an influenza
pandemic."1 With these words on November 1, 2005, President
George W. Bush introduced the National Strategy for Pandemic Influenza,
published by the Homeland Security Council. The motivation for its development
was the recent alarming events related to the avian influenza in Asia. In
addition, the pandemic potential of avian influenza has generated a
substantial amount of media publicity in recent months. Since pharmacists are
highly accessible to the general public, they will likely encounter many
questions regarding avian influenza. This article aims to provide a basic
understanding of the current issues surrounding avian influenza to assist
pharmacists' important role in public health education and vaccine
administration.
Influenza Virus
The virus
responsible for avian influenza is a subtype of the common influenza virus
that causes disease outbreak on an annual basis. Therefore, to develop an
understanding of avian influenza, it is helpful to begin with some background
information on the influenza virus itself.
The influenza virus is a
single-stranded RNA virus that belongs to the Orthomyxoviridae virus family
2 and is divided into three distinct types: influenza A, influenza B,
and influenza C. Of these three, type A is most often implicated in human
morbidity and mortality, followed by type B. The influenza C virus, however,
is not associated with serious disease in humans. Type A is further divided
into subtypes based on antigenic surface protein markers, whereas type B is
not divided into subtypes. The influenza A virus infects many animals,
including humans, cats, pigs, horses, sea mammals, and birds; the natural
reservoir for all known strains of type A is waterfowl.3,4 Type A
has a remarkable ability to undergo antigenic changes regularly, preventing
lifetime immunity to influenza in host organisms by vaccination or immune
response secondary to disease. This ability is largely due to a lack of
proofreading during viral replication, leading to a high rate of small
mutational errors. Overall, this may be viewed as an adaptive mechanism that
allows the influenza A virus subtype to evade host immune system defenses.
5
The most important antigenic
marker proteins on the surface of the influenza A virus are hemagglutinin (H)
and neuraminidase (N). Hemagglutinin is important for virus attachment to the
cells of the infected host, as well as for the release of viral genetic
material into the host cell. Once released, the viral RNA is used as a genetic
template for viral replication. If the animal host has immunity against
hemagglutinin, the chances for infection are reduced and disease severity is
decreased if disease develops. Neuraminidase is vital for the release of newly
synthesized virus from the host cells, allowing proliferation of the infection.
6 It is from these two antigenic protein markers that the H and N
designations of the influenza A virus subtypes are derived (e.g., H5N1).
Yearly mutations in these antigenic markers, a process called antigenic
drift, leads to modified strains of the type A subtypes that humans have
reduced immunity to. Of the known influenza A virus subtypes (H1 to H16 and N1
to N9), only three currently circulate among humans: H1N1, H1N2, and H3N2.
3
Influenza infection is an
important cause of morbidity and mortality in the United States during winter
months. In a typical year, about 36,000 people die from complications due to
influenza.7 The influenza virus is spread mostly by inhalation of
aerosolized respiratory droplets that are generated usually by the coughing
and sneezing of those infected.2,7
Human influenza that develops
into disease causes a wide spectrum of illness, from uncomplicated to
complicated influenza. Most patients with influenza experience an abrupt onset
of symptoms, such as fever, myalgia, nonproductive cough, sore throat,
headache, and malaise, that last about one to two weeks. Children, patients
over age 65, and patients with certain underlying medical conditions (e.g.,
cardiac disease, diabetes, chronic respiratory diseases) are at risk for
severe and possibly life-threatening complications from influenza Complicated
influenza that causes serious illness and death commonly occurs by coinfection
with other pathogens (viruses or bacteria), leading to pneumonia. Typical
influenza is due to influenza viruses that generally do not have the ability
to cause primary viral pneumonia but instead cause pneumonia indirectly by
facilitating the infection of lower respiratory tissue with other pathogens.
In stark contrast, avian influenza has a relatively high risk of causing
primary viral pneumonia that is associated with significant mortality.
Epidemiology of Influenza
In terms of
epidemiology, influenza may be classified as either epidemic or pandemic.
Influenza causes annual epidemics, where local geographic regions have
increased rates of morbidity and mortality secondary to influenza.
Fortunately, pandemics are far less common. Historically, pandemic influenza
arises every 10 to 30 years and entails a worldwide increase in morbidity and
mortality due to influenza that typically occurs in several waves.4
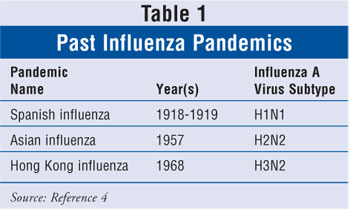
The pandemics of the previous century (table 1) have all been the result of
the influenza A virus. The greatest pandemic was the infamous 1918–1919
Spanish influenza in which an estimated 40 million people died worldwide.
When a major antigenic change occurs
in influenza type A, such that a new subtype is created to which an antibody
is lacking, pandemic influenza results.4 This phenomenon is known
as antigenic shift--a much more dramatic event in influenza epidemiology
than the smaller annual changes known as antigenic drift. With the
exception of the Russian influenza pandemic, past influenza pandemics have
been due to a new influenza A virus subtype that was both highly pathogenic
and readily spread by person-to-person transfer. The annual influenza vaccine
is modified to reflect antigenic changes caused by antigenic drift. However,
the emergence of a highly pathogenic influenza A subtype secondary to
antigenic shift, such as the virus causing avian influenza, will likely
require a considerable length of time for vaccine development. During this
time period, it is likely that pandemic influenza will occur.
The ability of the influenza
virus to infect and circulate in many types of animals allows high rates of
mutational change, leading to an increased chance of transferring new viral
subtypes to humans.8 Subtypes of the influenza A virus were not
known to spread from birds to humans until 1997. Prior to this time, it was
generally believed that the avian virus would initially spread to other
mammals (e.g., swine) that could be coinfected with a human strain of
influenza. In the swine, avian and human influenza strains could "swap" genes
in a process known as reassortment, allowing a novel humanized strain
to emerge. However, this assumption regarding influenza type A was altered by
the first major human exposure to avian influenza. Recent evidence indicates
two possible mechanisms by which avian influenza may acquire pathogenic
potential: (1) coinfection of a human host with an avian and human influenza
virus and subsequent gene reassortment, giving rise to a virus capable of
human-to-human transfer and (2) direct mutation of avian influenza to a virus
capable of human-to-human transfer.9 For example, the deadly H1N1
influenza virus that caused the 1918 influenza pandemic was likely developed
by the latter mechanism, as recent research findings have shown that this
virus arose from direct mutation of an avian influenza virus.10
Avian Influenza
Avian influenza
("bird flu") is a type of influenza virus carried in the intestines of wild
birds, especially waterfowl.11 Wild birds infected with this virus
do not typically get sick but can transmit it to domestic birds. Avian
influenza viruses can be devastating to domesticated birds. Due to the
conditions associated with the poultry industry, such as crowded bird
populations and humans living in close contact with these birds, ample
opportunity exists for people to interact with birds infected with avian
influenza. The immune system of the host organism recognizes the hemagglutinin
marker of influenza viruses to which it has been exposed by antibodies
produced by natural infection or vaccination. Thus, avian influenza may be
very devastating to humans if the viruses circulating among birds have
hemagglutinin markers that are not recognized by the human immune system. If
this virus is highly pathogenic to birds and could then attain the ability to
be transmitted from human to human, instead of just bird to human, then the
stage is set for a severe influenza pandemic.
In 1997, a strain of influenza
A (H5N1) that was highly pathogenic to poultry emerged in Hong Kong, resulting
in worldwide attention to avian influenza for the first time. Aside from the
pathogenic nature of this virus to poultry, public health officials found the
unprecedented ability of this virus to transfer from poultry to humans
especially alarming.12 A subtype of influenza A that was highly
pathogenic to poultry and previously unknown to infect humans had now
developed the ability to cause disease and death in humans. The outbreak began
with the discovery of avian influenza A (H5N1) in a 3-year-old boy who later
died of pneumonia, which was directly caused by the virus and complications of
Reye's syndrome. By the end of 1997, 18 individuals had been infected by the
virus; of these, six died of complications due to the infection.4
The massive culling or killing of all poultry in Hong Kong during the outbreak
has been credited as a potential reason that a pandemic was avoided.5
In the years since the initial outbreak, several additional subtypes of avian
influenza have been found to transfer from birds to humans, such as H9N2,
H7N3, and H7N7.4 Furthermore, several cases of probable
person-to-person transfer of avian influenza have recently been documented.
8 The following section discusses the H5N1 subtype, currently the most
important avian influenza subtype.
Of the avian influenza
subtypes documented as causing infection in humans, avian influenza A (H5N1)
is of greatest concern due to the high rates of infection in poultry and a
disturbing rise in morbidity and mortality in humans. Following the 1997
outbreak of the H5N1 virus in poultry and subsequent infection in humans, the
virus continued to circulate in animal reservoirs but was not reported to
cause disease in humans.13 In February 2003, human infection by
H5N1 was reported for the first time since 1997. From December 26, 2003, to
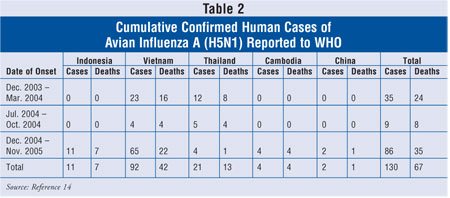
November 17, 2005, the World Health Organization (WHO) reported 130 cases of
human infection by the currently circulating H5N1 virus in five nations (table
2). Of these cases, over 50% of patients have died from influenza
complications.14 In addition to causing infection in humans, the
H5N1 infection continues to spread among birds, where it has been a cause of
significant health and economic devastation. For example, during 2004, H5N1
was reported in the poultry of nine Asian nations: Cambodia, China, Indonesia,
Japan, Laos, Malaysia, South Korea, Thailand, and Vietnam.15,16
In each of these outbreaks, culling around a certain radius of the affected
flock was used to protect humans and other flocks. Although this was a
necessary step, it created economic hardship on the affected farmers and,
ultimately, the entire industry.
Most human cases of avian influenza
A (H5N1) are believed to have been the result of contact with contaminated
bird excretions. The first case of probable person-to-person transfer of H5N1
was reported from the 2004 outbreak, which occurred in eight Asian nations.
8 In this case, a child (the index patient) became ill three to four
days after exposure to dying household chickens infected with the H5N1 virus.
The index patient's mother and aunt were subsequently infected while caring
for the child. Both the index patient and her mother died of pneumonia and
progressive respiratory failure within three hours (index patient) to several
days (patient's mother) after admission to the hospital. This case report
represents two important points regarding avian influenza. First, the case may
indicate the probable ability of the virus to transfer from infected poultry
to humans and also to transfer from person to person; however, sustained human
transmission has not been documented. Second, the virus' ability to cause
primary viral pneumonia is alarming because influenza usually facilitates the
development of pneumonia by other pathogens in patients at risk for
complications, rather than being a direct cause of pneumonia.
The clinical features of the
H5N1 infection in humans have similarities to and differences from those of
typical human influenza. The incubation period of the virus in humans is
likely two to five days, but some case reports indicate the incubation period
may be up to eight days.17 Initial symptoms of H5N1 include high
fever (>38ºC), cough, rhinorrhea, diarrhea, vomiting, abdominal pain,
shortness of breath, myalgia, and headache. During physical examination and
laboratory analysis, many patients present with pulmonary infiltrates,
lymphopenia, increased aminotransferase levels, and thrombocytopenia. Several
clinical features, such as lymphopenia and thrombocytopenia, are atypical of
classic uncomplicated influenza, especially in terms of their frequency of
occurrence in infected patients. The fact that nearly all patients present
with symptoms of primary viral pneumonia (often hemorrhagic) is especially
alarming, indicating that the influenza virus directly caused pneumonia--a
characteristic of influenza that is generally only seen during pandemics.
Thus, it is now clear that many deaths in the 1918–1919 outbreak were due to
primary viral pneumonia. Progression to acute respiratory distress syndrome
(ARDS), multiorgan failure, and sepsis syndrome is common and occurs within
four to 13 days after the onset of symptoms. Although the mortality rate has
been especially high among hospitalized patients, the mortality rate among all
patients is likely much lower. The death rate among infected children is
higher than it is in adults. For example, during a recent outbreak in
Thailand, the fatality rate was 89% among patients younger than 15 years.
18 Overall, death occurs within about nine to 10 days after onset of
symptoms, with most patients dying of progressive respiratory failure.
Interestingly, the response of
the human immune system to H5N1 may contribute to disease pathogenesis.17
Several immune factors, such as interleukin-6, tumor necrosis factor-alpha,
interferon-l, and soluble interleukin-2 receptor, were elevated in patients in
both 2003 and 2004 disease outbreaks. In addition, these immune factors have
been found in higher levels in patients who have died, compared to those who
have lived. It is hypothesized that elevation of these immune factors may lead
to the ARDS, sepsis, and multiorgan failure observed in victims.
To summarize, the avian
influenza A (H5N1) virus is a highly pathogenic subtype of influenza A that
causes extensive disease and death in domesticated birds. Outbreaks of H5N1
have occurred throughout Asia and, in rare cases, have resulted in
transmission to humans, causing infection and high rates of death. Whether
true person-to-person transfer of H5N1 has occurred remains uncertain, but it
is clear from previous bird flu pandemics (e.g., 1918 pandemic) that this can
occur.
Management of Avian
Influenza
Given the lack of
treatment options, the best methods to manage avian influenza are monitoring
and prevention. Several national and international health organizations, such
as the CDC and WHO, have emphasized the importance of continued efforts in
monitoring circulating avian influenza viruses and disease outbreaks in birds.
The prevailing opinion among experts is simple: If an outbreak is detected
that can efficiently spread from person-to-person before it has spread from
the region of origin, then a pandemic may be prevented by isolation of
infected individuals.1 Accordingly, several nations have initiated
strong collaborative support of the WHO global surveillance network. The CDC
is one of four WHO collaborating centers that provides support to the WHO
global surveillance network.19 Domestically, the National Influenza
Pandemic Preparedness Task Force, an interagency organized by the U.S.
Secretary for Health and Human Services, has been established to prepare the
nation for a possible influenza pandemic.
At this point, there is little
definitive information regarding effective management of patients with avian
influenza. No clearly effective medical treatment, other than supportive care,
has emerged during the management of infected persons during recent outbreaks
in Asia. Antiviral medications and corticosteroids are examples of medications
intuitive to the management of influenza; however, more evidence is needed to
support their use in a pandemic situation.14
Avian influenza A (H5N1)
remains highly sensitive in vitro to the neuraminidase inhibitors oseltamivir
(Tamiflu) and zanamivir (Relenza). However, the strains of H5N1 implicated in
recent human infection are uniformly resistant to amantadine and
rimantadine--influenza drugs that work by a different mechanism. Based on this
in vitro data, the WHO recommends that all persons with suspected avian
influenza A (H5N1) receive a neuraminidase inhibitor pending the results of
diagnostic testing. Specifically, the WHO recommends 75 mg of oseltamivir
twice daily for five days in adults and weight-adjusted twice-daily doses for
five days in children.17 For treatment of severe infection, the WHO
recommends higher doses of oseltamivir (150 mg twice daily for seven to 10
days in adults). Although the recent WHO recommendations do not include
zanamivir dosing in avian influenza, it should be noted that this
neuraminidase inhibitor has comparable activity against avian influenza
(H5N1), which may result in the use of zanamivir at doses equivalent to
oseltamivir in the event of an H5N1 outbreak.
Thus far, no currently
marketed vaccine provides immunity against any avian influenza A subtype that
has caused disease in humans. However, the current influenza vaccine may
provide indirect protection against the emergence of an avian influenza virus
with pathogenic potential. Theoretically, the current influenza vaccine would
decrease the chance of coinfection by avian and human influenza viruses by
preventing proliferation of human influenza viruses in hosts. This, in turn,
would decrease the chances of genetic reassortment that occurs between avian
and human influenza viruses and leads to an avian influenza virus with
pandemic potential. Accordingly, the CDC and WHO recommend that individuals in
possible contact with avian influenza, such as health care workers and certain
international travelers, receive the current influenza vaccine.20
In addition to the indirect protection provided by the current human influenza
vaccine, the National Institute of Allergy and Infectious Disease awarded
contracts to Sanofi-Pasteur and Chiron in 2004 for the development of avian
influenza vaccines.21 As a result, several vaccines against avian
influenza A (H5N1) are in progress. In one case, a vaccine based on H5N1
strains isolated during a 2004 outbreak in humans has been reported to be
immunogenic at high doses of hemagglutinin.17 The National
Institutes of Health expects to receive official reports from these trials in
2006.
Role of the Pharmacist
Given the number
and frequency of avian influenza–related news in the media, pharmacists will
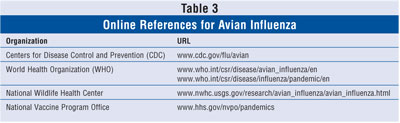
undoubtedly face questions from their patients regarding this disease. Several
excellent sources of online information about avian influenza are updated
regularly. Much of the information in this article will quickly become
outdated. Accordingly, the pharmacist seeking information regarding avian
influenza is encouraged to access relevant Web sites (table 3), which include
recommendations of health care agencies about the preparation for, and
management of, a possible influenza pandemic.
Pharmacists have a vital role in
public health by facilitating the safe and effective distribution of
medications. In the event of an outbreak of pandemic influenza, this role will
be even more important. Accordingly, several national pharmacy organizations
have collaborated with the federal government to create the Pharmacy National
Response Team (PNRT). If an outbreak of pandemic influenza occurs, it is
likely that services of the PNRT would be called upon. Pharmacists interested
in pursuing a direct role in the PNRT should consult the PNRT Web page at the
U.S. Department of Homeland Security National Disaster Medical System Web site
(ndms.fema.gov/nprt.html).
In recent years, the
pharmacist's role as a vaccine advocate has become increasingly important. In
addition to providing vaccine education to patients and promoting the use of
vaccines in patients who need them, pharmacists may now legally administer
several vaccines (including the influenza vaccine) in many states. Given the
potential for the current human influenza vaccine to protect against the
emergence of pandemic influenza, this role is now particularly significant.
The lack of an available vaccine for avian influenza may cause some patients
to believe that they have no reason to receive this year's influenza vaccine.
However, as in previous years, the total of vaccine-preventable human
influenza deaths in the U.S. this winter will most likely exceed all deaths in
humans from avian influenza to date. For these reasons, pharmacists should
encourage all candidates eligible for the influenza vaccine to be immunized.
(See www.cdc.gov/flu/professionals/vaccination for a complete list of persons
recommended to receive the influenza vaccine.) Also, pharmacists may wish to
consider becoming vaccine providers. (See www.immunize.org/laws/pharm.htm for
a list of states in which pharmacists are currently allowed to administer
vaccines.)
The absence of a vaccine and
proven treatment methods for avian influenza serves to heighten tensions in
the medical community and the general public regarding the threat of a
pandemic. Unfortunately, this anxiety has led to irrational behavior, like the
hoarding of antiviral medications. The news of oseltamivir activity against
avian influenza A (H5N1) in vitro, and the subsequent WHO recommendation for
use of oseltamivir in patients with suspected infection, has led to reports of
hoarding of this drug. However, the assumption that oseltamivir will be
efficacious against a potential avian influenza pandemic is dubious at this
point for several reasons. First, as already noted, there is no sound clinical
evidence of benefit derived from the use of oseltamivir in victims infected
with avian influenza. Second, influenza A viruses may become resistant to
neuraminidase inhibitors by a single point mutation in their viral RNA.
Considering the ability of the influenza virus to mutate, it is possible that
a future strain of a pandemic-causing avian influenza virus may be resistant
to this class of drugs.15 Indeed, the current strain of avian
influenza A (H5N1) implicated in recent human disease outbreaks is already
resistant to the older antiviral medications amantadine and rimantadine.
(Human influenza A in the U.S. remains generally sensitive to these
medications.) Also, in limited case reports, the virus has shown resistance to
oseltamivir.15 Third, hoarding of antiviral medications diverts
supply from public health agencies that need to create stockpiles so that
medication may be effectively distributed if an influenza pandemic occurs.
Finally, the unnecessary exposure of patients to drug therapy only serves to
increase their risk of unnecessary adverse effects. Thus, pharmacists can also
help discourage and prevent the hoarding of influenza antiviral medications.
Summary
If a new subtype of
the influenza virus to which we have no antibodies emerges, and it can infect
and spread in humans, then an influenza pandemic will ensue. Avian influenza
viruses that are highly pathogenic to domesticated birds are currently
circulating in Asia. Furthermore, several outbreaks of avian influenza in
humans have occurred secondary to the transmission of these viruses to humans.
Although it is uncertain, person-to-person transfer of avian influenza virus
may have occurred in isolated cases. Alarmingly, the mortality rate in humans
infected with avian influenza A (H5N1) is extraordinarily high, mostly due to
the virus' ability to cause primary viral pneumonia. These events are of great
concern, as humans do not have immunologic protection against these types of
viruses; therefore, the potential of a devastating pandemic is more likely now
than in the past. However, national and international efforts are under way to
prevent such a pandemic through coordinated surveillance, vaccine and
antiviral production, and public policy. Pharmacists are encouraged to remain
updated on this dynamic issue, as they may serve as a useful source of
information and provider of vaccination and medications to their patients.
REFERENCES
1. National
Strategy for Pandemic Influenza. Available at: www.whitehouse.
gov/homeland/pandemic-influenza.html. Accessed November 17, 2005.
2. Treanor JJ.
Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases
. 5th ed. Philadelphia, PA: Churchill Livingstone; 2000.
3. Hien TT, Liem NT,
Dung NT, et al. Avian Influenza A (H5N1) in 10 patients in Vietnam. N Engl
J Med. 2004;350(12):1179-1188.
4. Horimoto T, Kawaoka
Y. Influenza: Lessons from past epidemics, warnings from current incidents.
Nat Rev Micro. 2005;3(8):591-600.
5. Weir E. The changing
ecology of avian flu. CMAJ. 2005;173(8):869-870.
6. Moscona A.
Neuraminidase inhibitors for influenza. N Engl J Med.
2005;353(13):1363-1373.
7. Diagnosis of
Influenza. Available at: www.cdc.gov/flu/professionals/diagnosis. Accessed
November 21, 2005.
8. Ungchusak K,
Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of
avian influenza A (H5N1). N Engl J Med. 2005;352(4):333-340.
9. Russell CJ, Webster
RG. The genesis of a pandemic influenza virus. Cell.
2005;123(3):368-371.
10. Taubenberger JK,
Reid AH, Lourens RM, et al. Characterization of the 1918 influenza virus
polymerase genes. Nature. 2005;6(437):889-893.
11. Avian influenza:
General information. Available at: www.cdc.gov/flu/avian/gen-info/facts.htm.
Accessed November 21, 2005.
12. Claas EC, Osterhaus
AD, van Beek R, et al. Human influenza A H5N1 virus related to a highly
pathogenic avian influenza virus. Lancet. 1998;351:472-477.
13. Li K, Wang J, Smith
G, et al. Genesis of a highly pathogenic and potentially pandemic H5N1
influenza virus in eastern Asia. Nature. 2004;430:209-213.
14. Cumulative number
of confirmed human cases of avian influenza A/(H5N1) reported to WHO.
Available at: www.who.int/csr/disease/
avian_influenza/country/cases_table_2005_11_17/en/index.html. Accessed
November 21, 2005.
15. The World Health
Organization Global Influenza Program Surveillance Network. Evolution of H5N1
avian influenza viruses in Asia. Emerg Infect Dis.
2005;11(10):1515-1521.
16. Tiensin T,
Chaitaweesub P, Thaweesak S, et al. Highly pathogenic avian influenza H5N1,
Thailand, 2004. Emerg Infect Dis. 2005;11(11):1664-1672.
17. The Writing
Committee of the World Health Organization (WHO) Consultation on Human
Influenza A/H5. Avian influenza A (H5N1) infection in humans. N Engl J Med
. 2005;353(13):1374-1385.
18. Chotpitayasunondh
T, Ungchusak K, Wanna H, et al. Human disease from influenza A (H5N1),
Thailand, 2004. Emerg Infect Dis. 2005;11(2):201-209.
19. Avian Influenza
Outbreaks in Asia. Available at: www.cdc.gov/flu/avian/outbreaks/asia.htm.
Accessed November 21, 2005.
20. Centers for Disease
Control and Prevention. Interim Recommendations for Infection Control in
Health-Care Facilities Caring for Patients with Known or Suspected Avian
Influenza. Available at: www.cdc.gov/flu/avian/
professional/infect-control.htm. Accessed December 15, 2005.
21. National Institute
of Allergy and Infectious Diseases. Questions and Answers H5N1 Avian Flu
Vaccine Trials. Available at: www3.niaid.nih.
gov/news/newsreleases/2005/H5N1QandA.htm. Accessed December 15, 2005.
To comment on this article,
contact
editor@uspharmacist.com.






