US Pharm. 2021;46(1):44-45.
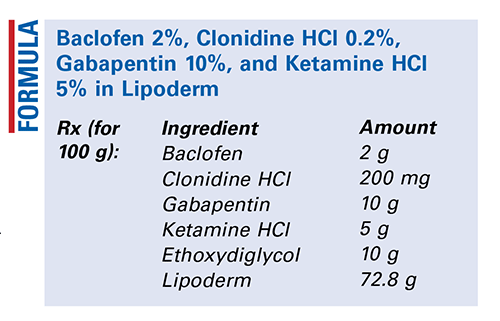
Method of Preparation: Calculate the quantity of each ingredient needed. Accurately weigh or measure each ingredient. Combine all the powders and mix well. Add the ethoxydiglycol and mix to form a smooth paste. Incorporate into the Lipoderm and mix well. Package in a calibrated dose-delivery device and label.
Use: This preparation has been used in the treatment of several neuropathic disorders, including restless legs syndrome (RLS).
Packaging: Package in a tight, light-resistant, child-resistant container. Use a calibrated dose delivery device.
Labeling: Keep out of reach of children. Keep refrigerated. Discard after ____ [time period]. For external use.
Stability: A beyond-use date of up to 30 days may be used for this preparation.1
Quality Control: Quality-control assessment can include theoretical weight compared with actual weight, specific gravity, active drug assay, color, texture, appearance, feel, rheologic properties, and physical observations.2,3
Discussion: RLS is a condition that causes uncomfortable leg sensations that have been described as tingly, crawling, or creeping and as a strong urge to move the affected limb. RLS is more common in women than in men, and it typically occurs while sitting, resting, or sleeping. RLS is mild in most patients, but it can be moderate to severe and may have an impact on daily activities, as it can disrupt sleep or rest and cause serious sleep problems. A number of different drugs and drug combinations have been used to treat RLS, including this preparation.
Baclofen (Lioresal, C10H12ClNO2, MW 213.66) occurs as a white to off-white, crystalline powder that is odorless or practically odorless. It is slightly soluble in water. The drug has pKa values of 5.4 and 9.5.1,4
Clonidine hydrochloride (HCl) (Catapres, Duraclon, C9H9Cl2N3.HCl, MW 266.55) occurs as a white to off-white, crystalline powder that is odorless and has a bitter taste. It is stable in light, air, and heat and does not exhibit polymorphism. Clonidine HCl is soluble 1 g in about 13 mL water and 25 mL alcohol. It is classified as a centrally acting alpha 2 adrenergic agonist and imidazoline receptor agonist and has been in clinical use since 1966.4
Gabapentin (cyclohexaneacetic acid, Neurontin, C9H17NO2, MW 171.24) occurs as a white to off-white, crystalline solid that is freely soluble in water and in alkaline and acidic solutions. A 2% aqueous solution has a pH of 6.5 to 8.0. Gabapentin is an anticonvulsant that is structurally related to gamma-aminobutyric acid. It is used in combination with other anticonvulsant agents in the management of seizure disorders, neuropathic pain and vasomotor symptoms, RLS, and other disorders.1,5
Ketamine HCl (C13H16ClNO.HCl, MW 274.2) is used as an anesthetic, as well as an analgesic. It occurs as a white, crystalline powder with a slight characteristic odor. Approximately 1.15 mg of ketamine HCl is equivalent to 1 mg of ketamine base. Ketamine HCl is soluble 1 g in 4 mL of water, 14 mL of alcohol, and 60 mL of absolute alcohol.1,5
Other names for ethoxydiglycol (C6H14O3, CH2OHCH2OCH2CH2OC2H5, MW 134.20) include diethylene glycol monoethyl ether, diethylene glycol ethyl ether, Carbitol, and Transcutol. This substance occurs as a colorless liquid with a mild pleasant odor. Ethoxydiglycol is hygroscopic, and it is miscible with water and with common organic solvents. It has a density of 1.027 and is combustible. Ethoxydiglycol is nonirritating and is used as a solvent, solubilizer, and cosurfactant.6
Lipoderm is an elegant alternative to traditional Pluronic Lecithin Organogels (PLOs), having a smooth and creamy feel rather than the tacky feel of PLOs. It contains a proprietary liposomal component that may increase the permeation of a variety of actives. Lipoderm is a stable system that does not separate upon refrigeration and has great resiliency in the presence of ionic substances.7
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; December 2020.
2. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 1: physical and chemical testing. IJPC. 2019;23(3):211-216.
3. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 2: microbiological testing. IJPC. 2019;23(4):299-303.
4. McEvoy GK, ed. AHFS Drug Information 2016. Bethesda, MD: American Society of Health-System Pharmacists; 2016:1489-1493,1887-1895.
5. Sweetman SC, ed. Martindale: The Complete Drug Reference. 36th ed. London, England: Pharmaceutical Press; 2009:523-526,1905-1906.
6. Ash M, Ash I. Handbook of Pharmaceutical Additives. Brookfield, VT: Gower Publishing Ltd; 1995:484.
7. Professional Compounding Centers of America. LIPODERM® (30-3338). www.pccarx.com/Products/ProductCatalog.aspx?pid=30-3338. Accessed December 2, 2020.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





