US Pharm. 2023;48(10):8-12.
ABSTRACT: The use of statins is common in hyperlipidemia therapy and for reducing atherosclerotic cardiovascular disease risk. However, patients who are statin intolerant—due to the therapeutic failure of a maximally tolerated dose—or experience adverse effects do not have an oral option that targets cholesterol synthesis in the body. Bempedoic acid is a novel oral cholesterol-lowering drug that targets cholesterol synthesis and only activates in the liver, which prevents common adverse effects associated with statins, such as muscle pain.
Hypercholesterolemia is a lipoprotein disorder in which the low-density lipoproteins (LDL) in the blood are above normal concentrations.1 LDL, synonymous with LDL-cholesterol (LDL-C), is naturally circulating in the blood and binds to LDL receptors to store cholesterol in the cells, which include arterial walls.2 Too much LDL-C can cause atherosclerotic cardiovascular disease (ASCVD), which can lead to fatal cardiovascular events; hypercholesterolemia pharmacotherapy aims to lower LDL-C concentrations.2,3 Adenosine triphosphate citrate lyase (ACLY) is an enzyme in the liver that plays a role in the synthesis of cholesterol and is a precursor to hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, which is targeted by statin drugs.4
Bempedoic acid is a first-in-class innovative prodrug approved by the FDA in February 2022 for the treatment of familial hypercholesterolemia (FH) or ASCVD.5 This prodrug is converted by liver enzyme ACSVL1 to bempedoyl-CoA, which inhibits ACLY and upregulates the AMP-activated protein kinase pathway. This results in synergistic downregulation of enzymes such as HMG-CoA reductase that increase LDL-C synthesis. Thus, it decreases LDL-C in the blood and lowers the risk of ASCVD.6
CLEAR Trials
The results of the CLEAR Harmony and Wisdom phase III trials allowed the FDA to approve the use of bempedoic acid to lower cholesterol in patients with hypercholesterolemia. Both trials were randomized, double-blind, and placebo-controlled. The trial periods were both 52 weeks in length and the trials compared the use of bempedoic acid 180 mg with placebo. The trials enrolled adults with ASCVD, with heterozygous FH, or who were taking the highest-intensity statin tolerated, excluding simvastatin 40 mg or higher.7-9
Clinical Safety
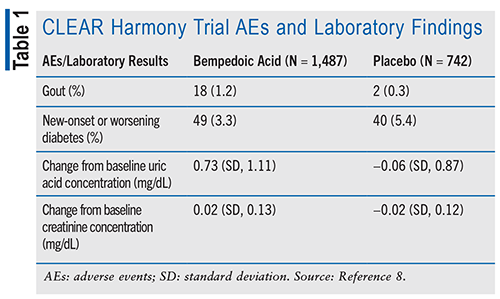
The FDA was primarily informed by the results of the CLEAR Harmony trial that bempedoic acid met the safety standards to be approved for public marketing. CLEAR Harmony included 2,230 patients in a 2:1 ratio of those receiving bempedoic acid and a placebo group.8 The researchers aimed to assess the overall safety via incidence of adverse effects and changes in safety laboratory results, such as estimated glomerular filtration rate, creatine kinase, creatinine, uric acid, and liver-function concentrations.8 Researchers found that the incidence of adverse events did not differ between patients who received bempedoic acid (78.5%) and the placebo group (78.7%).8 Patients in the bempedoic acid group had less incidence of new-onset or worsening diabetes compared with placebo. Nasopharyngitis, myalgia, upper respiratory tract infection, urinary tract infection, arthralgia, dizziness, muscle spasms, and diarrhea were common adverse events, occurring with similar frequency in both groups.8 At Week 12, the incidence of gout was significantly higher in the bempedoic acid group compared with placebo.8 In addition, one significant laboratory finding was that baseline uric acid concentrations increased slightly in the bempedoic acid group, while the other findings were insignificant. Although the incidence of serious adverse events was generally small and similar in the two groups, the percentage of patients who discontinued the trial due to an adverse event was higher in the bempedoic acid group than in the placebo group (10.9% vs. 7.1%).8 TABLE 1 displays a few significant adverse events and laboratory findings.
Clinical Efficacy
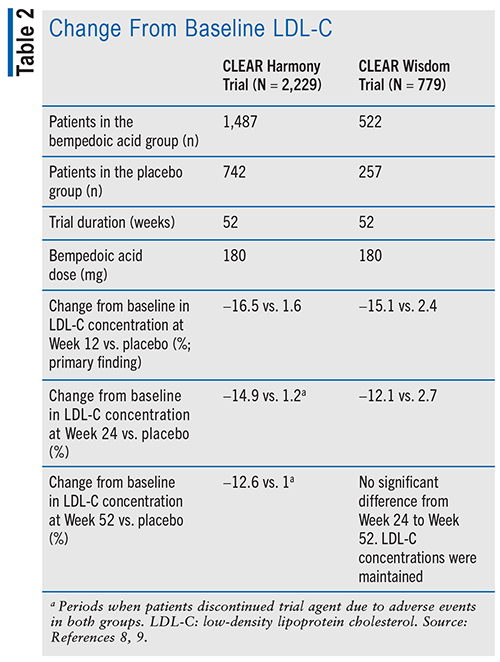
Additionally, the CLEAR Harmony trial assessed the percent change in LDL-C concentrations at Week 12 as the principal efficacy endpoint. Researchers found that bempedoic acid statistically and clinically reduced the mean LDL-C concentration by 19.2 mg/dL (a decrease of 16.5% from baseline), while patients in the placebo group did not have any significant decrease.8 Although LDL-C concentrations were recorded for the entire duration of the trial, trial discontinuations occurred after Week 12. Additionally, researchers found that non–high-density lipoprotein cholesterol (non-HDL-C), total cholesterol, apolipoprotein B, and high-sensitivity C-reactive protein in patients who received bempedoic acid improved significantly when compared with placebo.
In CLEAR Wisdom, 779 patients were assigned in a 2:1 ratio similarly to CLEAR Harmony.9 Researchers mirrored the primary endpoint from CLEAR Harmony but further recorded changes from baseline LDL-C concentrations at Weeks 24 and 52 as their secondary and tertiary endpoint, respectively. At Week 12, patients who received bempedoic acid experienced a significant 15.1% reduction from baseline LDL-C concentrations, compared with a 2.4% increase in LDL-C in placebo patients.9 TABLE 2 compares the percent change from baseline LDL-C concentrations found in both trials. Furthermore, improvements in lipid parameters and biomarkers such as non-HDL-C, total cholesterol, triglycerides, apolipoprotein B, and high-sensitivity C-reactive protein were maintained through Week 52.9
Impact on Cardiovascular Outcomes
Although the primary purpose of this drug is to lower LDL-C concentrations, cardiovascular outcomes play a vital role in whether providers would want to prescribe this medication. CLEAR Outcomes—a double-blind, randomized, placebo-controlled trial—assessed death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, and coronary revascularization in 13,970 patients for approximately 40.6 months.10 Researchers found that these events were significantly lower in patients who were given bempedoic acid.10 Similar results were also found in the previous CLEAR trials (Harmony and Wisdom), in which bempedoic acid improved cardiovascular outcomes.8,9 This verifies that bempedoic acid is associated with a decreased risk of major cardiovascular events, and community pharmacists do not need to be overly wary of dispensing this new medication.
Significant Adverse Effects
Gout was found to be a common adverse event across the three CLEAR trials mentioned.8-10 This is attributed to the increase in serum uric acid concentrations associated with bempedoic acid.11 The mechanism is caused by how bempedoic acid competes with uric acid for the organic anion transporter 2 in the liver during metabolism.6,12 This causes competitive inhibition, preventing uric acid from being excreted from the body, thus elevating blood concentrations and possibly causing hyperuricemia. Furthermore, bempedoic acid is associated with tendon rupture, a rare adverse event found in CLEAR Harmony and Wisdom.8,9 However, all of the tendon ruptures or injuries occurred in patients taking moderate- or high-dose statins and non–low-dose statins.12
Drug-Drug Interactions
Concurrent administration of simvastatin 20 mg with bempedoic acid 240 mg or pravastatin 40 mg with bempedoic acid 180 mg was found to increase serum concentrations of these statins.12 Statins may cause serious muscle-related adverse effects, such as myalgia, weakness, cramps, and rhabdomyolysis.13 No other significant drug-drug interactions were found. Counseling patients on the need to monitor for these adverse events is an important function for pharmacists.
Place in Therapy
In hypercholesterolemia therapy, statins have been the primary option in reducing LDL-C concentrations to the desired goals recommended to reduce ASCVD risk.14 When alternative or additional therapy is required, there are other options, such as ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.15 Ezetimibe is an affordable oral option added to a maximally tolerated statin therapy when the LDL-C concentrations remain greater than 70 mg/dL. If LDL-C concentrations are not in goal range after a maximally tolerated statin and ezetimibe, PCSK9 inhibitors can be added.16 Nonetheless, many patients taking ezetimibe do not reach their recommended therapeutic LDL-C goal.14 Also, the use of PCSK9 inhibitors, such as alirocumab or evolocumab, out of reach for many patients due to high cost and difficulty in receiving insurance prior authorization.17 A patient survey conducted in community practice found that 74.6% of patients found the drug approval process to be burdensome, and out-of-pocket costs were the leading reported reason for discontinuation.18
Bempedoic acid is indicated as an adjunctive pharmacologic option to diet and a maximally titrated statin to treat hypercholesterolemia or ASCVD that requires LDL-C concentration–lowering therapy.19 The recommended dosage is 180 mg taken orally once daily with or without food.19 Although bempedoic acid is not teratogenic, it is not recommended in pregnancy and lactation due to its mechanism of action in decreasing cholesterol synthesis.19 Thus, bempedoic acid should be discontinued immediately for pregnant or breastfeeding patients.
Cost
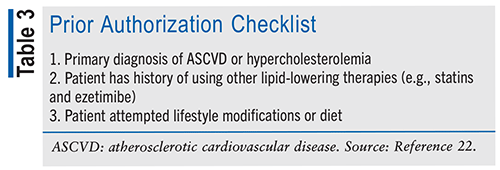
The cost of a month’s supply of bempedoic acid 180-mg tablets is approximately $394 with a GoodRx savings coupon.20 However, patients can apply for a copay card on the manufacturer’s website, lowering the cost to $10 per month.21 Patients who are uninsured or have government-provided insurance—such as Medicare Part D and Medicaid—are not eligible for the copay card.21 Patients who are on government programs such as Medicare Part D can have their physician request a prior authorization.22 TABLE 3 shows the required criteria to be authorized.
Conclusion
Statin therapy is the primary treatment for hypercholesterolemia, and other efficacious nonstatin options come in an injectable form. Bempedoic acid is the first-in-its-class oral nonstatin cholesterol synthesis inhibitor option that is effective in lowering LDL-C and decreasing the risk of ASCVD. It is a last-line option for patients who have trouble controlling their cholesterol with maximally titrated statins or who cannot tolerate them. Pharmacists can work with physicians in providing the best individualized cholesterol-lowering therapy for patients to treat hypercholesterolemia and lower their risk of ASCVD.
REFERENCES
1. Ibrahim MA, Asuka E, Jialal I. Hypercholesterolemia. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. www.ncbi.nlm.nih.gov/books/NBK459188/.
2. Pirahanchi Y, Sinawe H, Dimri M. Biochemistry, LDL cholesterol. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. www.ncbi.nlm.nih.gov/books/NBK519561/.
3. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Circulation. 2019;140(11):e546-e646.
4. Feng X, Zhang L, Xu S, et al. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: an updated review. Prog Lipid Res. 2020;77:101006.
5. Tummala R, Gupta M, Devanabanda AR, et al. Bempedoic acid and its role in contemporary management of hyperlipidemia in atherosclerosis. Ann Med. 2022;54(1):1287-1296.
6. Biolo G, Vinci P, Mangogna A, et al. Mechanism of action and therapeutic use of bempedoic acid in atherosclerosis and metabolic syndrome. Front Cardiovasc Med. 2022;9:1028355.
7. FDA. Drug trials snapshots: Nexletol. March 2, 2020. www.fda.gov/drugs/resources-information-approved-drugs/drug-trials-snapshots-nexletol. Accessed September 7, 2023.
8. Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380(11):1022-1032.
9. Goldberg AC, Leiter LA, Stroes ESG, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom randomized clinical trial. JAMA. 2019;322(18):1780-1788.
10. Nissen SE, Lincoff AM, Brennan D, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388:1353-1364.
11. Cicero AFG, Pontremoli R, Fogacci F, et al. Effect of bempedoic acid on serum uric acid and related outcomes: a systematic review and meta-analysis of the available phase 2 and phase 3 clinical studies. Drug Safety. 2020;43(8):727-736.
12. Ballantyne CM, Bays H, Catapano AL, et al. Role of bempedoic acid in clinical practice. Cardiovasc Drugs Ther. 2021;35(4):853-864.
13. Attardo S, Musumeci O, Velardo D, et al. Statins neuromuscular adverse effects. Int J Mol Sci. 2022;23(15):8364.
14. Agarwala A, Quispe R, Goldberg AC, et al. Bempedoic acid for heterozygous familial hypercholesterolemia: from bench to bedside. Drug Des Devel Ther. 2021;15:1955-1963.
15. Abdul-Rahman T, Bukhari SMA, Herrera EC, et al. Lipid lowering therapy: an era beyond statins. Curr Probl Cardiol. 2022;47(12):101342.
16. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25).
17. Baum SJ, Toth PP, Underberg JA, et al. PCSK9 inhibitor access barriers-issues and recommendations: improving the access process for patients, clinicians and payers. Clin Cardiol. 2017;40(4):243-254.
18. Bradley CK, Shrader P, Sanchez RJ, et al. The patient journey with proprotein convertase subtilisin/kexin type 9 inhibitors in community practice. J Clin Lipidol. 2019;13(5):725-734.
19. Nexletol (bempedoic acid) tablets, film-coated package insert. Ann Arbor, MI: Esperion Therapeutics, Inc; 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/211616s000lbl.pdf.
20. GoodRx. Nexletol (bempedoic acid): uses, side effects, dosage & more. www.goodrx.com/nexletol/what-is. Accessed July 23, 2023.
21. Esperion Therapeutics. Resources: Nexlizet (bempedoic acid and ezetimibe) and Nexletol (bempedoic acid). www.nexlizethcp.com/resources. Accessed July 23, 2023.
22. Esperion Therapeutics. Common criteria for submission of a NEXLIZET or NEXLETOL prior authorization (PA) form. www.nexlizethcp.com/content/pdf/prior_authorization_checklist_update_(digital).pdf. Accessed July 23, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.





