US
Pharm. 2006;5:62-69.
Osteonecrosis of the jaws
(ONJ) is a serious oral condition that has been reported more frequently by
dentists and physicians in the last few years in cancer patients receiving
chemotherapy, radiation, corticosteroids, or other cancer treatment regimens
concomitant with the use of bisphosphonates. These patients may also present
with comorbid conditions such as anemia, infections, and preexisting oral
disease. Although the original published reports of ONJ involved intravenous
bisphosphonates, currently some cases with oral bisphosphonates are occurring.
Patients with ONJ usually present with oral signs and symptoms of painful,
exposed, and necrotic bone, primarily of the mandible and to a lesser extent
the maxilla, after dental treatment. This article reviews the development of
osteonecrosis of the jaws, potential risk/precipitating factors, the
pharmacology of bisphosphonates, and potential preventive measures for this
oral complication.
Background
Introduced in the
mid-1990s, bisphosphonates were prescribed as an alternative to hormone
replacement therapies for osteoporosis and to treat osteolytic tumors and
possibly slow tumor development. In 1996, Fosamax (alendronate) was the first
bisphosphonate drug approved for osteoporosis (in which low bone mass and
reduced bone strength lead to fractures of the spine, wrist, and hip) in
postmenopausal women (and later, in men); it was approved later on for
treatment of Paget's disease (in which normal bone is replaced with
poor-quality bone).
The first studies on the
actions of bisphosphonates to block calcification and bone destruction were
published in the 1960s. Didronel (etidronate) was first used over 20 years ago
to treat patients with osteoporosis, but they developed osteomalacia and
clinical studies were stopped. In the United States, Didronel is not approved
for treatment of osteoporosis.
In 2005, the Food and Drug
Administration approved Boniva (ibandronate sodium) as the first once-a-month
tablet for postmenopausal osteoporosis. Studies showed that it significantly
reduced the risk of new vertebral fractures and increased bone mineral density.
1 In January 2006, the FDA approved Boniva injection, the first
injectable medication for treatment of postmenopausal osteoporosis.
Injectables were developed for individuals having difficulty with oral
bisphosphonate dosing requirements, including an inability to sit upright for
30 to 60 minutes and/or to swallow a pill. Additionally, the injectable
product is administered once every three months by a physician so that
monitoring is possible.
The FDA has notified the
dental/medical community to the potential problem of osteonecrosis of the jaws
(also referred to as avascular or aseptic necrosis)--primarily of the mandible
but also cases of the maxilla--occurring in association with bisphosphonates.
The first reported cases were associated with intravenous bisphosphonates used
to control hypercalcemia in metastatic bone disease2 but there are
also anecdotal unpublished reports of oral bisphosphonates causing ONJ.
Drug Properties
Bisphosphonates
are synthetic analogues of pyrophosphates, identified in the 1960s as
substances present in blood and urine that prevent the formation and
aggregation of calcium phosphate crystal.3 Pyrophosphates are used
in tartar control toothpastes to inhibit the formation of tartar (calculus)--a
hard calcium deposit on the tooth. Bisphosphonates were developed because
pyrophosphates were rapidly metabolized in the body. Figure 1 shows the basic
chemical structure of bisphosphonates.
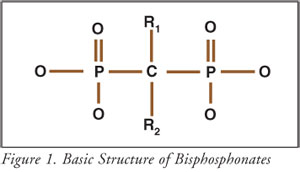
Bisphosphonates are not metabolized; only about 3% of the drug is absorbed after an oral dose.4 Within 24 hours, about half the absorbed dose is excreted in the urine and the rest is distributed to bone, from which it is slowly eliminated.
Mechanism of Action
The function of bisphosphonates is
still relatively unclear. Bisphosphonates bind and accumulate in bone and
remain there for months after therapy is discontinued. They are potent
inhibitors of normal and abnormal osteoclastic bone resorption that results in
metastatic bone disease.5 They also reduce the local release of
factors that stimulate tumor growth (antitumor effect).6
Bisphosphonates are known to inhibit osteoclast attachment to bone, to induce
apoptosis (programmed cell death) of osteoclasts, and to inhibit
differentiation of bone marrow precursor cells into osteoclasts; they may also
contribute to inhibition of bone resorption and increase in bone mass.3
Although they block bone resorption, formation continues for about six to 12
months, after which formation stops. Thus, the mineral is more densely packed
so that the bone density will increase even though the bone volume does not.
Bisphosphonates also act on
osteoblasts to inhibit osteoclast activity and osteoclastic function, an
integral part of the normal turnover and maintenance of bone. Osteoclastic
function is so severely impaired that osteocytes are not replaced and the
capillary network in the bone is not maintained. This results in avascular
bone necrosis. The net effect is that physiologic bone deposition and
remodeling are severely compromised in cancer patients receiving
bisphosphonates.7 The length of drug exposure may play a role in
the development of ONJ.8 Patients taking bisphosphonates for more
than six months are at the highest risk for ONJ.9
Formulations and
Indications
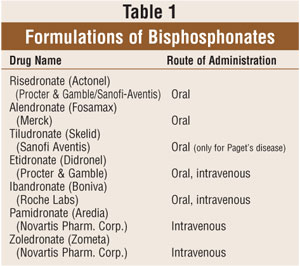
Table 1 lists the
oral and parenteral bisphosphonate products currently available in the U.S.
Bisphosphonates are indicated for both the treatment and prevention of
osteoporosis and in cancers metastatic to bone (skeletal complications of
malignancy).6 See Table 2.
Alendronate and risedronate are
oral preparations used to treat osteoporosis. Zoledronic acid, pamidronate,
and ibandronate are the only intravenous bisphosphonates that are indicated
for treatment of hypercalcemia of malignancy, such as squamous cell carcinoma
of the head and neck, breast cancer, prostate cancer, multiple myeloma, and
renal cancer. The incidence of hypercalcemia in cancer patients is about 20%
to 30%; the prognosis is very poor, and many patients die. The
first-generation oral bisphosphonates, such as alendronate and risedronate,
are not useful in the treatment of hypercalcemia of malignancy because they
are not as potent as the second- and third-generation intravenous drugs.
Intravenous bisphosphonates are also used with standard antineoplastic therapy
in the treatment of breast, lung, and prostate cancer metastatic to bone to
prevent bone complications and in multiple myeloma by interfering with bone
marrow activities (through inhibition of osteoclastic activity).

Risk Factors for Osteonecrosis
of the Jaws
Documented risk
factors for osteonecrosis of the jaws are listed in Table 3. As mentioned
earlier, it is unknown if dental extraction/surgery is a cause of ONJ or just
a precipitating or exacerbating factor that hastens bone necrosis. Invasive
dental procedures that may precipitate osteonecrosis include tooth
extractions, placement of dental implants, and periodontal surgery. Even
pressure from dentures, which results in local mucosal infections beneath the
denture, may be followed by bone involvement.

Adverse Events: Reported Cases of Osteonecrosis of the Jaws
Wang et al. reported the first
cases of ONJ associated with bisphosphonate therapy in cancer patients.2
These cancer patients were undergoing many treatments with chemotherapy
drugs, corticosteroids, and bisphosphonates. Marx10 reported on a
group of 36 patients receiving intravenously either pamidronate or zoledronate
for the management of bone disease associated with metastatic cancer, multiple
myeloma, and osteoporosis, who had developed avascular necrosis of the jaws.
In the majority of cases, the necrosis developed after tooth extractions
(nonhealing extraction sites), and in about 30% of cases it occurred
spontaneously.
Instances of osteonecrosis
of the jaw bones have been reported for both injectable and oral
bisphosphonates and may be a class effect, according to the FDA, as exhibited
by alendronate, zoledronic acid, and pamidronate cases. In a 2005 report, Marx
et al.11 studied 119 cases of bisphosphonate-associated bone
exposure in patients taking Aredia (26%), Zometa (40.3%) and Fosamax (3%). The
mean time for bone to be exposed in the oral cavity with accompanied symptoms
was 14.3 months. Precipitating events that caused the bone exposure were as
follows: 37.8% tooth extractions, 28.6% advanced periodontal disease, 25.2%
spontaneous, 11.2% periodontal surgery, 3.4% placement of dental implants, and
0.8% root canal surgery.11
Clinical Features of
Osteonecrosis of the Jaws
The exact
mechanism underlying this reaction is unknown; however, it has been postulated
that bisphosphonates inhibit new vessel formation in the bone, which is
associated with absent or delayed hard (alveolar) and soft tissue healing,
usually after dental extractions.12,13 The oral lesions seen in ONJ
appear similar to those of radiation-induced osteonecrosis.13,14
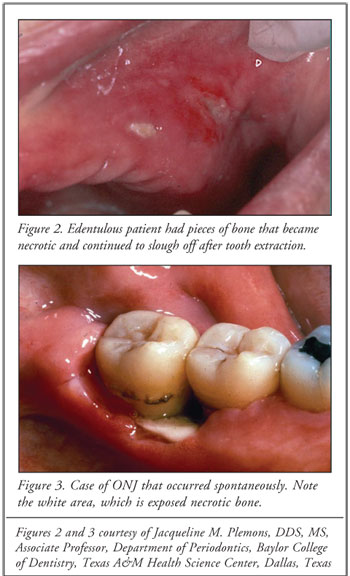
Clinically, there is oral ulceration with exposed underlying necrotic ("dead")
bone (Figures 2 and 3). This oral condition causes chronic pain and severe,
irreversible dysfunction and disfigurement of the jaw. Other symptoms include
soft tissue swelling, infection, and mobility of teeth.
Patients may remain
asymptomatic for many weeks or months, and ONJ may be recognized only by the
presence of exposed "painful" bone in the mouth. These lesions most likely
become symptomatic when the necrotic sites become secondarily infected or if
there is trauma to the soft tissue.
Although dental surgery or
extractions have been identified as precipitants in many of these cases, as
ONJ developed after tooth extraction, there is evidence suggesting that the
jaw (alveolar) bone can be involved before and independent of dental
procedures.15 The condition can develop spontaneously.
Treatment Management
Treatment
management involves educating the dentist (periodontist, oral surgeon,
prosthodontist), pharmacist, physician, and patient about ONJ and preventive
measures that need to be taken to avoid these oral complications. The American
Academy of Oral Medicine published a position paper addressing the prevention
of bisphosphonate-associated osteonecrosis and the dental care management of
patients with cancer and/or osteoporosis who are taking bisphosphonates.12
In 2005, The American Academy of Periodontology (AAP) published a statement
on bisphosphonates, making periodontists aware of the need to determine if
patients are currently taking intravenous bisphosphonates or if any patients
will be treated with these drugs. The AAP stresses the importance of
identifying ONJ and other oral complications of cancer and cancer therapy.
The FDA and drug companies
have published statements for dental health professionals regarding the
development of ONJ in patients being treated for cancer with intravenous
bisphosphonates. In late 2004, for example, Novartis had implemented changes
to Zometa and Aredia product labels to include precautions on osteonecrosis of
the jaws. The precaution states that a dental exam and preventive dentistry
should be considered prior to treatment with bisphosphonates in patients with
concomitant risk factors such as cancer, chemotherapy, corticosteroids, and
poor oral hygiene. In February 2005, the FDA released a statement that ONJ is
a risk of all bisphosphonates, not just the IV formulations.
Prior to starting on
bisphosphonate therapy, patients should be counseled regarding the possible
occurrence of ONJ.16 If possible, invasive dental procedures should
be avoided while patients are taking the medications. It is recommended that
dentists perform a thorough soft tissue and dental examination before a
patient starts using bisphosphonate drugs. If bisphosphonate therapy can be
briefly delayed without the risk of skeletal-related complications, dental
procedures should be performed on the patient who requires root canal therapy
or tooth extraction, denture adjustment, periodontal surgery, or placement of
dental implants.17 Avoiding tooth extractions while patients are
taking bisphosphonates should minimize the incidence of ONJ.18 Once
bisphosphonate therapy has begun, the dentist should monitor the patient's
oral health on a regular basis. Early detection is the key.
The pharmacist should
counsel the patient taking a bisphosphonate on self-care oral hygiene. Some
recommendations include using a soft-bristled toothbrush, replacing the
toothbrush frequently to maintain its shape and effectiveness, and avoiding
alcoholic over-the-counter mouthrinses since the alcohol will "dry" oral
tissues.
Dental consultation before
initiating bisphosphonate therapy is essential. Dental health and maintenance
of oral tissues are extremely important for cancer patients taking
bisphosphonates. Pharmacists and dentists should report suspected cases of ONJ
to the FDA's MedWatch Adverse Event Reporting Program online at
www.fda.gov/medwatch/report.htm or by phone at 1-800-FDA-1088.
Mouthrinses, systemic
antibiotics, hyperbaric oxygen, and surgical debridement have been tried as
treatments for ONJ, but no treatment has been proven effective. Marx et al.
11 found that a combination of antibiotics and 0.12% chlorhexidine
mouthrinse was about 90% effective in controlling pain in patients with
painful exposed bone. Discontinuing the bisphosphonate is not recommended once
necrosis of the jaws has occurred.19 Focus should be placed on
prevention--the patient should have regular dental examinations and any
invasive dental treatment done before bisphosphonate therapy begins.
Individuals using oral or
intravenous bisphosphonates have contact with their pharmacist. Some may
report to the pharmacist that they have oral pain or bleeding from inside
their mouth. After consulting with such patients about their medications and
dental visits, the pharmacist should refer the patient to the dentist and/or
physician.
Summary
The pharmacist,
dentist, and physician all play a pivotal role in the patient's chemotherapy
regimen. The pharmacist and dentist are in the best positions to educate the
patient about the potential adverse events that can occur once starting
bisphosphonates and of the necessary preventive measures. Complete prevention
of ONJ is not currently possible.11 Therefore, the patient must
maintain excellent oral hygiene to reduce the risk of dental and periodontal
infections. Patients should be advised to have routine dental examinations
before and during cancer/bisphosphonate treatment and to report any oral
symptoms to their dentists and physicians promptly.
REFERENCES
1. America's Bone
Health: The State of Osteoporosis and Low Bone Mass in Our Nation: The
National Osteoporosis Foundation; February 2002.
2. Wang J, Goodger
NM, Pogrel MA. Osteonecrosis of the jaws associated with chemotherapy. J Oral
Maxillofac Surg 2003;61:1104-1107.
3. Fleisch H.
Bisphosphonates--history and experimental basis. Bone 1987;8:Suppl 1:S23-28.
4. Fleish H.
Bisphosphonates: pharmacology and use in the treatment of tumour-induced
hypercalcaemic and metastatic bone disease. Drugs 1991;42(6):919-944.
5. Licata AA.
Discovery, clinical development, and therapeutic uses of bisphosphonates. Ann
Pharmacother 2005;39:668-677.
6. Green JR.
Antitumor effects of bisphosphonates.Cancer.2003;97(3 suppl):840-847.
7. Odvina CV, Zerwekh
JE, Rao DS, et al. Severely suppressed bone turnover: a potential complication
of alendronate therapy. J Clin Endocrinol Metab 2005;90:1294-1301.
8. Bamias A,
Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after
treatment with bisphosphonates: incidence and risk factors. J Clin Oncol
2005;23(24):8580-8587.
9. Ruggiero SL,
Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated
with the use of bisphophonates: a review of 63 cases. J Oral Maxillofac Surg
2004;62:527-534.|
10. Marx RE.
Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of
the jaws: a growing epidemic. J Oral Maxillofac Surg 2003;61:1115-1117.
11. Marx RE, Sawatari
Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone
(osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition
prevention, and treatment. J Oral Maxillofac Surg 2005 Nov;63(11):1567-1575.
12. Migliorati CA,
Casiglia J, Epstein J, et al. Managing the care of patients with
bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine
position paper. J Am Dent Assoc. 2005;136(12):1658-1668. Erratum in: J Am Dent
Assoc. 2006;137(1):26.
13. Migliorati CA,
Schubert MM, Peterson DE, Seneda LM. Bisphosphonate-associated osteonecrosis
of mandibular and maxillary bone: an emerging oral complication of supportive
cancer therapy. Cancer 2005;104:83-93.
14. Melo MD,Obeid G.
Osteonecrosis of the jaws in patients with a history of receiving
bisphosphonate therapy. Strategies for prevention and early recognition. J Am
Dent Assoc 2005;136:1675-1681.
15. Purcell PM, Boyd
IW. Bisphosphonates and osteonecrosis of the jaw. Med J Aust 2005;182:417-418.
16. Zarychanski R,
Elphee E, Walton P, Johnston J. Osteonecrosis of the jaw associated with
pamidronate therapy. Am J Hematol 2006;81:73-75.
17. Katz H.
Endodontic implications of bisphosphonate-associated osteonecrosis of the
jaws: a report of three cases. J Endod 2005;31(11):831-834.
18. Gibbs SDJ,
O'Grady J, Seymour JF, Prince HM. Bisphosphonate-induced osteonecrosis of the
jaw requires early detection and intervention. Med J Aust 2005;183(10):549-550.
19. Lenz JH,
Steiner-Krammer B, Schmidt W, et al. Does avascular necrosis of the jaws in
cancer patients only occur following treatment with bisphosphonates? J
Craniomaxillofac Surg 2005;33(6):395-403.
To comment on this article,
contact editor@uspharmacist.com.






