US Pharm. 2022;47(7):29-33.
Breastmilk is the natural source of nutrition for infants. It is an essential source of nutrients for infants, containing water, fat, proteins, and lactose. Breastfeeding has been linked with a reduced risk for many illnesses, such as otitis media and respiratory and gastrointestinal tract infections in infants, as well as a lower risk for sudden infant death syndrome, childhood obesity, and diabetes. For mothers, breastfeeding decreases the risk of premenopausal breast cancer, ovarian cancer, type 2 diabetes, and high blood pressure. Breastfeeding should be considered a public health concern rather than a lifestyle choice; Healthy People 2030 include objectives to increase the proportion of infants who are breastfed exclusively through age 6 months and to increase the proportion of infants who are breastfed at 1 year. The American Academy of Pediatrics (AAP) recommends that all infants be exclusively breastfed for the first 6 months of life, followed by continued breastfeeding as complementary foods are introduced for 1 year or longer, as mutually desired by mother and infant. According to the National Immunization Survey, 84% of infants were breastfed in 2018, with only 46.3% and 25.8% of infants being exclusively breastfed through 3 and 6 months.1-5
One barrier to breastfeeding is the mother’s concern about taking medications while breastfeeding. Women who are breastfeeding are more likely to take medications when they are breastfeeding compared with when they are pregnant. Almost 96% of breastfeeding mothers use one or more medications while breastfeeding, and many will follow “when in doubt, don’t breastfeed,” causing disruptions in breastfeeding and the introduction of formula feeding. These brief disruptions can result in negative consequences on lactation, potentially decreasing the supply of milk, sensitizing the infant’s immune system, and increasing the risk of many diseases with the early introduction of cow’s milk. Pharmacists are the most accessible healthcare professional and frequently encounter breastfeeding mothers. They can serve as a source of support to this patient population, encouraging the continuation of breastfeeding and alleviating medication and lactation concerns.6,7
Classification of Drug Risk
The “Nursing Mothers” subsection of the previously FDA-approved labeling often did not provide enough information to make an informed decision about the potential exposure of medication via breastmilk to the infant. In 2008, the FDA published a proposed regulation on the format and content requirements for pregnancy and lactation labeling. This new labeling provides information about the risks and benefits of medication use during pregnancy and lactation in a format that is consistent. The new “Lactation” section provides information about the use of the medication while breastfeeding, including the amount of drug in breastmilk and the potential adverse effects on the infant. Information to help minimize exposure to the infant and any recommended dose adjustments, if known, will also be provided. Finally, all safety data supporting the recommendations will also be summarized so that healthcare professionals can make a more informed decision when weighing the risk versus benefit of using the medication.8,9
Medication Safety
Healthcare providers have very little information to help determine the potential risks and benefits to both mother and child due to the lack of randomized clinical trials in this patient population. Information about the extent of drug excretion in breastmilk or drug exposure to the child via breastmilk may be unavailable or is based on data from animal studies. Human data found in drug labeling and the literature are mainly derived from case studies and case series. To fully determine the potential of a medication affecting a breastfeeding infant, many factors need to be taken into consideration, including the clinical pharmacology and pharmacokinetic profiles of the medication.
The transfer of medication into the breastmilk is driven largely by passive diffusion. Drug concentration in the milk is largely determined by maternal serum drug concentration, but factors such as low molecular weight, high lipophilicity, low protein binding, and a basic pH all increase the likelihood of medication transfer into breastmilk. The ratio of drug concentrations in breastmilk-to-drug concentrations in maternal plasma, also known as the milk-to-plasma ratio, can be a useful tool to help determine the relative degree of drug excretion into breastmilk. However, this does not provide information about the safety of the medication in breastfeeding. Most medications have a milk-to-plasma ratio of 1 or less, indicating that the drug does not accumulate in the breastmilk. The relative infant dose (RID) provides an estimation of infant drug exposure. This value compares the infant dose received via breastmilk relative to the mother’s dose and is expressed as a percentage. In general, an RID of less than 10% is considered acceptable, and the degree of drug exposure in breastmilk is considered clinically unimportant.10-13
Based on the pharmacokinetic and pharmacodynamic considerations discussed above, some general recommendations can be made to guide drug selection in lactating mothers. It would be prudent to select medications with short half-lives, high-protein binding, low oral bioavailability, or high molecular weights. Furthermore, utilizing drugs with an RID of less than 10% is preferred. All of these considerations would help to minimize the extent of drug exposure to the feeding infant.10-13
Lactation Resources
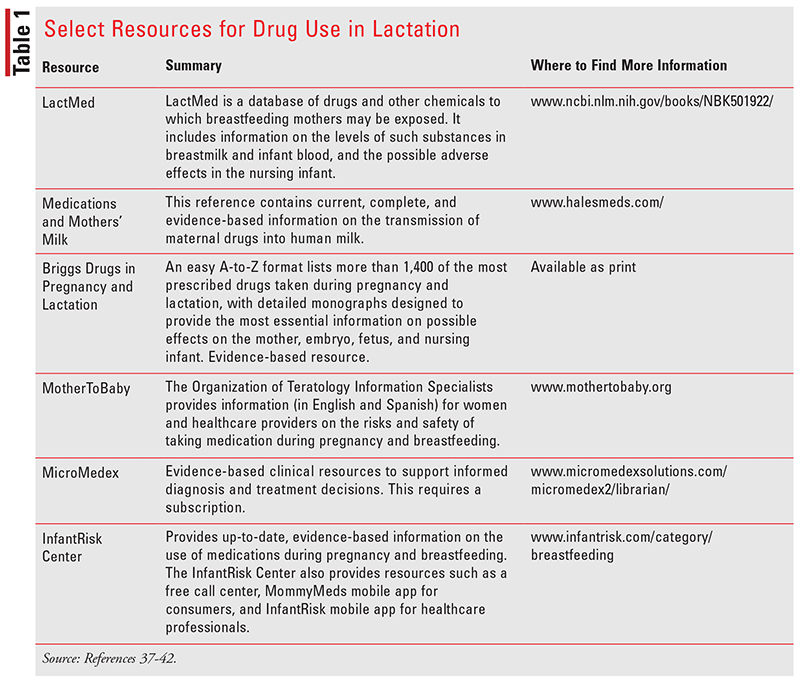
Several resources are available to aid pharmacists and other healthcare professionals in evaluating the appropriateness of medication usage in lactating mothers (see TABLE 1). When utilizing these resources, it is important to bear in mind that there may be variability in the safety recommendations obtained from these resources. This variability may be due in part to the number of references cited by the resources to make their safety determinations; LactMed, an Internet-based database published by the National Library of Medicine/National Institutes of Health, has been cited as utilizing the greatest number of resources with an average of 15.1 references per monograph, as compared with 5.6 references with MicroMedex and 5.3 references with Medications and Mothers’ Milk. It is also important to realize that these references differ in how often the information is updated. For example, the print version of Drugs in Pregnancy and Lactation is published every 3 years, but the electronic version is updated every 3 months. Of all the references listed, the AAP regards LactMed as the most comprehensive and up-to-date resource regarding the use of medications during lactation.14
OTC Medications and Breastfeeding
As many women who are breastfeeding may seek assistance in selecting OTC medications for the self-treatment of common conditions, it is essential that pharmacists be familiar with the general safety recommendations of common classes of nonprescription drug products. Generally, products with published safety data are preferred in this patient population over newer products that may not have been used extensively in lactating women.
Analgesics
Analgesics are some of the most commonly used OTC products, with several different ingredients available for patient use. Acetaminophen and ibuprofen are the products that are considered safe to use during breastfeeding and are commonly recommended. Both products have favorable pharmacokinetic profiles and have limited excretion into breastmilk, which makes them suitable for use in lactating mothers. Although naproxen is a propylamine belonging to the same category as ibuprofen, it is generally not recommended for use in breastfeeding due to its longer half-life. The use of naproxen has been documented to cause prolonged bleeding, anemia, and thrombocytopenia in an infant whose mother had been using the medication during breastfeeding.15-18
Aspirin is excreted into breast milk as salicylic acid, with higher doses resulting in disproportionately higher concentrations. Long-term, high-dose aspirin therapy should be avoided, and alternative analgesics should be utilized. Daily low-dose aspirin therapy can be considered for those requiring cardiovascular protection. However, breastfed infants should be monitored for signs of bruising and bleeding.19
Allergy and Cold Products
All of the currently available OTC antihistamines are excreted to some extent in breastmilk. These agents also have anticholinergic properties that can potentially decrease milk production and make breastfeeding difficult. The use of sedating antihistamines, which include first-generation agents and cetirizine, can also cause lethargy or irritability in breastfed infants. To minimize this risk, nonsedating antihistamines are preferred for short-term use.20
Oral decongestants available for OTC use include pseudoephedrine and phenylephrine. Oral phenylephrine has poor oral bioavailability and is unlikely to adversely affect a feeding infant. However, there is limited information regarding the safe use of this agent in lactation, and it is therefore not recommended for use. There is more information available regarding the use of pseudoephedrine during breastfeeding. Small amounts of the drug are excreted in breastmilk and may cause some degree of irritability in the nursing infant. However, a greater concern is that pseudoephedrine has been associated with decreased milk production, especially with repeated use. As such, the drug should be avoided in mothers who are having difficulty producing enough milk. Alternatively, topically applied oxymetazoline may be used; however, its use should be limited to no more than 3 days due to the risk of developing rhinitis medicamentosa.21,22
Cough and cold products commonly include dextromethorphan as a cough suppressant and guaifenesin as an expectorant. Dextromethorphan is found in breastmilk in very low concentrations and is not expected to have adverse effects in breastfeeding infants. Limited data are available regarding the use of guaifenesin in lactating women, and it should therefore be avoided.23,24
Gastrointestinal Products
Several ingredients are available OTC for the management of diarrhea, including loperamide. Although the drug has not been extensively studied in lactating women, it is generally considered acceptable for use during lactation because of its minimal oral bioavailability. Bismuth subsalicylate is not recommended because of the possible absorption of salicylate from breastmilk.25,26
Various classes of agents are available for the prevention and management of constipation. Bulk laxatives, such as psyllium, are not systemically absorbed and are considered safe to use while breastfeeding. Stimulant laxatives, such as bisacodyl and senna, are generally considered to be safe to use during breastfeeding, although there have been some reports of increased frequency of loose stools in breastfed infants. The stool softener docusate is minimally absorbed, and it is therefore unlikely to be found in breastmilk.27-29
Common medications used for the management of gastroesophageal reflux disease and related symptomatology include H2-blockers, proton-pump inhibitors, and antacids. All currently available H2-blockers available OTC are considered safe to use during breastfeeding. Cimetidine should be considered the least desirable agent to use because of its extensive drug interaction profile. Limited information is available regarding the use of proton-pump inhibitors during lactation, but the limited concentration of these agents in breastmilk is unlikely to have any harmful effects on the feeding infant. Because of their local action within the gastrointestinal tract and their limited systemic absorption, no special precautions are required when antacids are used by a mother who is breastfeeding.30-36
Conclusion
The benefits of breastfeeding for both the mother and infant are widely known and supported by all major healthcare organizations and published guidelines. Although many new mothers may wish to breastfeed their infants, their concerns regarding the safety of medication use during breastfeeding may discourage them from doing so. Their fears may lead concerned mothers to avoid breastfeeding altogether or to not use recommended medications essential to their health. Pharmacists, as medication experts who are readily available to the public, are in a unique position to counsel mothers regarding both the benefits of breastfeeding as well as the safe use of medications during lactation. Several comprehensive resources and databases are now available for pharmacists to access the most current safety information regarding both prescription and OTC medications as they provide essential patient education to lactating mothers. Because many OTC products for common conditions are considered safe to use during breastfeeding, pharmacists can counsel patients on proper product selection to ensure good patient outcomes for both the mother and child.

Should I Consider Breastfeeding?
Breastfeeding is recommended by the American Academy of Pediatrics. They recommend that all children be exclusively breastfed for the first 6 months, and when foods are slowly introduced, breastfeeding should continue up to at least the baby’s first birthday.
Why Should I Breastfeed My Baby?
Besides providing the necessary nutrients to your newborn, breastfeeding has been associated with a decreased risk for infections, including ear infections and respiratory infections. Breastfeeding also provides protection against sudden infant death syndrome, childhood obesity, and diabetes. It is easier to digest than formula. Breastfeeding provides you with some benefits as well. It makes it easier for you to lose some of the weight you gained during pregnancy. Breastfeeding may also decrease your risk of ovarian and breast cancer, diabetes, and high blood pressure.
Can I Breastfeed if I Am Taking Medications?
You should not use medications while breastfeeding unless it is needed to improve your health. Not treating an illness can be harmful. However, it is important to understand that both OTC and prescription medications may pass into your breastmilk, though most medications get into your milk at low levels and are considered safe in most instances. There are exceptions, and some drugs can pass into your milk and have an effect on your baby. The amount of drug present in milk is dependent on the type of drug, the dose, and the level of drug in your body when your baby feeds. There may be more than one type of medication that can treat your condition. Your pharmacist or other healthcare providers will be able to assist you in choosing the safest medication for you and your baby.
How Can I Minimize the Risk to My Baby When Using a Medication?
You should always inform your healthcare providers that you are breastfeeding. Do not utilize an OTC medication without first consulting your pharmacist to ensure that it is safe to use while breastfeeding. If you are taking medications, try feeding your baby just before you take the medication to limit the amount of drug that is passed into your breastmilk. The amount of drug in your milk should be at its lowest at this time. Limit or avoid alcohol and smoking while breastfeeding. Illegal drugs and medications prescribed to other individuals should not be used and have the potential to cause great harm.
Where Can I Get More Information?
It is always important for you to speak with your pharmacist or healthcare provider when you have questions regarding the safety of drugs when breastfeeding. Some websites you can refer to include:
National Library of Medicine: www.medlineplus.gov/breastfeeding.html
Centers for Disease Control and Prevention: www.cdc.gov/breastfeeding/
Remember, if you have questions, Consult Your Pharmacist.
REFERENCES
1. Duale A, Singh P, Al Khodor S. Breast milk: a meal worth having. Front Nutr. 2022;8:800927.
2. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827-e841.
3. Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2(4):222-231.
4. Health.gov. Increase the proportion of infants who are breastfed exclusively through age 6 months—MICH‑15. www.health.gov/healthypeople/objectives-and-data/browse-objectives/infants/increase-proportion-infants-who-are-breastfed-exclusively-through-age-6-months-mich-15. Accessed June 15, 2022.
5. Health.gov. Increase the proportion of infants who are breastfed at 1 year—MICH‑16. www.health.gov/healthypeople/objectives-and-data/browse-objectives/infants/increase-proportion-infants-who-are-breastfed-1-year-mich-16. Accessed June 15, 2022.
6. Stultz EE, Stokes JL, Shaffer ML, et al. Extent of medication use in breastfeeding women. Breastfeed Med. 2007;2(3):145-151.
7. Akus M, Bartick M. Lactation safety recommendations and reliability compared in 10 medication resources. Ann Pharmacother. 2007;41(9):1352-1360.
8. Federal Register. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. May 29, 2008. www.federalregister.gov/documents/2008/05/29/E8-11806/content-and-format-of-labeling-for-human-prescription-drug-and-biological-products-requirements-for. Accessed June 10, 2021.
9. Federal Register. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. December 4, 2014. www.federalregister.gov/documents/2014/12/04/2014-28241/content-and-format-of-labeling-for-human-prescription-drug-and-biological-products-requirements-for. Accessed June 10, 2021.
10. Spencer JP, LS III Gonzalez, Barnhart DJ. Medications in the breast-feeding mother. Am Fam Physician. 2001;64(1):119-126.
11. Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343(2):118-126.
12. Wang J, Johnson T, Sahin L, et al. Evaluation of the safety of drugs and biological products used during lactation: workshop summary. Clin Pharmacol Ther. 2017;101(6):736-744.
13. Chaves RG, Lamounier JA. [Breastfeeding and maternal medications]. J Pediatr Rio J. 2004;80(5 Suppl):S189-S198.
14. Holmes AP, Harris JB, Ware S. Evaluation of lactation compatibility reference recommendations. Ann Pharmacother. 2019;53(9):899-904.
15. National Library of Medicine. Acetaminophen. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501194/. Accessed June 16, 2022.
16. National Library of Medicine. Ibuprofen. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK500986/. Accessed June 16, 2022.
17. National Library of Medicine. Naproxen. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501044/. Accessed June 16, 2022.
18. Fidalgo I, Correa R, Gómez Carrasco JA, Martínez Quiroga F. [Acute anemia, rectorrhagia and hematuria caused by ingestion of naproxen]. An Esp Pediatr. 1989;30(4):317-319.
19. National Library of Medicine. Aspirin. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501196/. Accessed June 16, 2022.
20. National Library of Medicine. Diphenhydramine. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501878/. Accessed June 16, 2022.
21. National Library of Medicine. Phenylephrine. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501438/. Accessed June 16, 2022.
22. National Library of Medicine. Pseudoephedrine. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501085/. Accessed June 16, 2022.
23. National Library of Medicine. Dextromethorphan. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501456/. Accessed June 16, 2022.
24. National Library of Medicine. Guaifenesin. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501455/. Accessed June 16, 2022.
25. National Library of Medicine. Loperamide. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501297/. Accessed June 16, 2022.
26. National Library of Medicine. Bismuth subsalicylate. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501324/. Accessed June 16, 2022.
27. National Library of Medicine. Psyllium. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501346/. Accessed June 16, 2022.
28. National Library of Medicine. Senna. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501349/. Accessed June 16, 2022.
29. National Library of Medicine. Bisacodyl. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501323/. Accessed June 16, 2022.
30. National Library of Medicine. Cimetidine. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501206/. Accessed June 16, 2022.
31. National Library of Medicine. Famotidine. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501267/. Accessed June 16, 2022.
32. National Library of Medicine. Ranitidine. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501252/. Accessed June 16, 2022.
33. National Library of Medicine. Nizatidine. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501240/. Accessed June 16, 2022.
34. National Library of Medicine. Omeprazole. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501242/. Accessed June 16, 2022.
35. National Library of Medicine. Esomeprazole. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501284/. Accessed June 16, 2022.
36. National Library of Medicine. Lansoprazole. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501283/. Accessed June 16, 2022.
37. National Library of Medicine. In: Drugs and Lactation Database (LactMed). 2006. www.ncbi.nlm.nih.gov/books/NBK501922/. Accessed June 16, 2022.
38. HalesMeds.com. Hale’s medications & mothers’ milk. www.halesmeds.com/. Accessed June 16, 2022.
39. Briggs GG, Freeman RK, Towers C, Forinash AB. Briggs Drugs in Pregnancy and Lactation. https://shop.lww.com/Briggs-Drugs-in-Pregnancy-and-Lactation/p/9781975162375. Accessed June 16, 2022.
40. MotherToBaby. www.mothertobaby.org/. Accessed June 16, 2022.
41. Micromedex. www.micromedexsolutions.com/micromedex2/librarian. Accessed June 16, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






