US Pharm. 2020;45(3):34-38.
ABSTRACT: Cannabidiol (CBD) is a nonpsychoactive component of the Cannabis sativa plant. CBD products, which have become popular in the United States, are frequently used to treat pain, anxiety, and sleep disorders—conditions that affect older adults. Evidence is insufficient to recommend the use of CBD for these disease states. OTC CBD products are widely available, and there are significant concerns regarding their safety, including mislabeling, standardization issues, and drug interactions. The informed pharmacist will be a valuable resource for discussing the use and safety of CBD with older adults.
Cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC) are among the many cannabinoids, or components, of the Cannabis sativa plant. CBD is nonpsychoactive, whereas THC has psychoactive properties such as euphoria and psychosis. Two common strains of Cannabis sativa are marijuana and hemp.1 CBD may be derived from either marijuana (which often contains more than 15% THC) or hemp (having a THC concentration of no more than 0.3%).2 In addition, CBD may be extracted from Cannabis indica and hybrid plants, which may have higher concentrations of CBD than THC. A recent survey revealed that one in seven Americans uses CBD products, with the most common reasons for its use being pain, anxiety, poor sleep, and arthritis.3
Endogenous cannabinoids and phytocannabinoids such as CBD and THC modulate the endocannabinoid system (ECS). THC is a partial agonist on the cannabinoid (CB) 1 receptor that results in central nervous system (CNS) effects, such as the “high” associated with marijuana; it also has limited CB2 agonist activity in the immune system. CBD has minimal activity on CB receptors, but it affects the ECS and the non-ECS.4 Some of the proposed mechanisms of CBD include agonist activity at serotonin 1A, transient receptor potential vanilloid 1, G protein–coupled receptor 55, and adenosine A2A receptors, which may explain some of the possible analgesic, anti-inflammatory, anxiolytic, and antiepileptic effects of CBD.1,5
In the United States, about two-thirds of states have legislation approving cannabis for recreational use (11 states) and/or medicinal purposes (21 additional states). Seven states mandate pharmacist involvement, such as dispensing activities or consulting to dispensaries.6 The only FDA-approved (in 2018) CBD product is Epidiolex, which is indicated for treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome.7 The law prohibits the sale of foods or dietary supplements to which CBD has been added; however, a wide variety of products containing CBD are available at retail stores.8
Formulations
CBD formulations used in clinical trials include oral capsules, sublingual spray, oil-based solution, and topical gel. OTC CBD products are available in numerous other formulations, including topical balms and creams, e-liquid for inhalation, and infused foods and drinks.1,9 Given the many formulations and manufacturers, nearly all CBD products lack standardization. The exception is Epidiolex, which is available as an oil-based oral solution formulated with sesame oil and standardized to contain 100 mg/mL of pure CBD extract.7
CBD levels in commercially available products vary widely. The FDA has issued warning letters every year since 2015 to companies marketing unapproved new drugs that allegedly contain CBD.8,10-13 As part of these warnings, the FDA tests the chemical content of CBD compounds, and it has found that many products do not contain the claimed level of CBD. Commercially available products have been assessed in laboratories, whose findings support the FDA’s concerns about product inconsistency and mislabeling. A laboratory assessment of OTC CBD products sold in the U.S. demonstrated that only 26 of 84 (31%) products tested were accurately labeled.14 Not only was the amount of CBD in products overlabeled or underlabeled, but 21% of products contained THC even though it was not listed in the product information. In addition, the FDA has cited concerns regarding reports of contaminants such as pesticides and heavy metals.8
The mislabeling of CBD products results in dosing uncertainty in the use of any commercially available OTC product. This is an important caveat in the extrapolation of dosages used in clinical research. In such research, a range of dosages have been used for different indications and routes of administration. For example, Epidiolex oral solution is approved for weight-based dosing from 5 mg/kg/day to a maximum of 20 mg/kg/day.7 CBD has been given orally at dosages of 100 mg to 800 mg.15,16 CBD topical gel has been used for fragile X syndrome at a dosage of 50 mg to 250 mg daily.17 For smoking cessation, a CBD metered-dose inhaler has been administered at a dosage of 400 mcg as needed.18
Administration and Absorption
CBD absorption depends on the product formulation. In animal and human studies, CBD administered orally has been shown to be poorly absorbed, with bioavailability of 13% to 19%.19,20 CBD’s bioavailability is believed to be reduced by first-pass metabolism. Poor bioavailability can be avoided with the use of alternative formulations. There is an emerging market for novel delivery methods to increase CBD’s oral bioavailability.21
Absorption of CBD may also be altered by food intake. In clinical trials, coadministration of Epidiolex with a high-fat, high-calorie meal increased plasma levels of CBD fourfold to fivefold compared with administration on an empty stomach.7 In one study using a purified (99%) CBD capsule, coadministration with food resulted in a maximum concentration and AUC of 14-fold and fourfold higher, respectively, compared with administration on an empty stomach.22 CBD inhalation in humans has an average bioavailability of approximately 31%, with the use of one type of metered-dose inhaler demonstrating bioavailability of more than 65%.18,23 Transdermal absorption of CBD is variable in animal studies and has yet to be fully elucidated in humans.4
CBD Uses
CBD is FDA-approved for certain types of seizure disorders; for more information, see the manufacturer’s website for Epidiolex (www.epidiolex.com). The following section will focus on the common reasons for off-label CBD use, including pain, sleep disorders, and anxiety, all of which affect older adults.
Pain: An estimated 50 million American adults (20.4%) experience chronic pain, with persons aged 65 years and older constituting 61.2% of those affected.24 Much of the data on chronic pain (e.g., neuropathic pain, cancer pain, diabetic peripheral neuropathy, fibromyalgia, HIV-associated sensory neuropathy, spasticity associated with multiple sclerosis [MS], and rheumatoid arthritis) involve the use of marijuana and cannabinoids (often THC, combination THC-CBD, or nabiximols [a specific mixture of THC, CBD, other minor cannabinoids, flavonoids, and terpenes]). Formulations used in pain studies range from smoked, oral, or oromucosal spray of THC; synthetic cannabis (nabilone); synthetic THC (dronabinol); and vaporized cannabis, with results suggesting modest reductions in pain and spasticity.25
Sativex (nabiximols), an oromucosal THC-CBD spray, is approved in several European countries for treating symptoms of moderate-to-severe spasticity associated with MS, and a phase II/III clinical trial is currently under way in the U.S. to evaluate nabiximols for advanced cancer pain with inadequate analgesia from chronic opioids.26 There is a paucity of data on CBD used for pain; most studies are in preclinical stages.5,25,27
Sleep Disorders: Sleep disorders are disproportionately more prevalent in older adults.28 Patients have commonly reported that cannabis is helpful for sleep.29 CBD is used for alleviation of insomnia, but little is known about its effectiveness. One study that compared CBD with placebo for insomnia in 15 patients suggested that 160 mg of CBD may improve sleep duration without next-day sedation.30 Somnolence was reported in nearly one-third of patients taking Epidiolex in clinical trials, which provides additional support for CBD’s benefits for sleep in some patients.7 However, more research is needed to determine whether CBD is useful for individual components of insomnia, such as sleep latency, wakefulness after sleep onset, sleep duration, and overall sleep quality.
Anxiety: Evidence is not strong for the use of CBD for anxiety disorders. CBD has demonstrated some benefit for social anxiety disorder and social phobia when patients undergo a simulated public-speaking test.31,32 However, these trials had small sample sizes and study biases. It is theorized that CBD could be beneficial for anxiety based on its mechanism of action at the serotonin receptor.31
Other Disease States: Data on the use of CBD for various other conditions are mixed, and evidence is insufficient to recommend this practice. The efficacy of CBD has been studied in bipolar disorder, Crohn’s disease, diabetes, dystonia, fragile X syndrome, graft-versus-host disease, Huntington’s disease, opioid withdrawal, Parkinson’s disease, schizophrenia, and smoking cessation.33 In addition, CBD has been reported to be useful for addiction, possibly by modulating dopamine and serotonin.1
Adverse Effects and Safety
The use of CBD is considered “possibly safe” when used appropriately, based on some clinical evidence.33 However, insufficient high-quality data exist to recommend CBD for most older adults. The most common adverse effects associated with CBD, reported in clinical trials of Epidiolex, are somnolence (~32%), decreased appetite (16%-22%), diarrhea (9%-20%), and increased liver-function tests (13%).7 Other side effects are orthostatic hypotension, lightheadedness, and dry mouth. Adverse effects appear to be dose-related. The safety of CBD in the geriatric population has not been fully clarified, and Epidiolex clinical trials did not include patients older than 55 years.7
There are practical concerns regarding CBD use in older adults. The geriatric population may be more susceptible to adverse effects of CBD commonly seen in younger adults, including sedation. CBD is hepatically metabolized, predominantly via CYP2C19 and CYP3A4.4 Older adults with reduced hepatic function may be more susceptible to adverse effects of CBD.
Commercially available CBD products may not contain the CBD concentrations claimed on the label, and the FDA warns consumers to be aware of this inconsistency when using such products.13 Of particular concern is the THC component in mislabeled CBD products. Older adults may be predisposed to adverse effects caused by the psychoactive properties of THC. The use of marijuana in older adults has been associated with increased risk of injury and adverse events.34
Drug-Drug Interactions
CBD has been shown to inhibit hepatic enzymes.4 In human studies, coadministration of CBD with antiepileptic drugs resulted in increased concentrations of drugs that are substrates of CYP2C9, CYP2C19, and CYP3A4.35 Given CBD’s known sedative effect, there is also a theoretical concern for additive hypnotic reactions in combination with CNS depressants. TABLE 1 lists potential interactions with CBD.
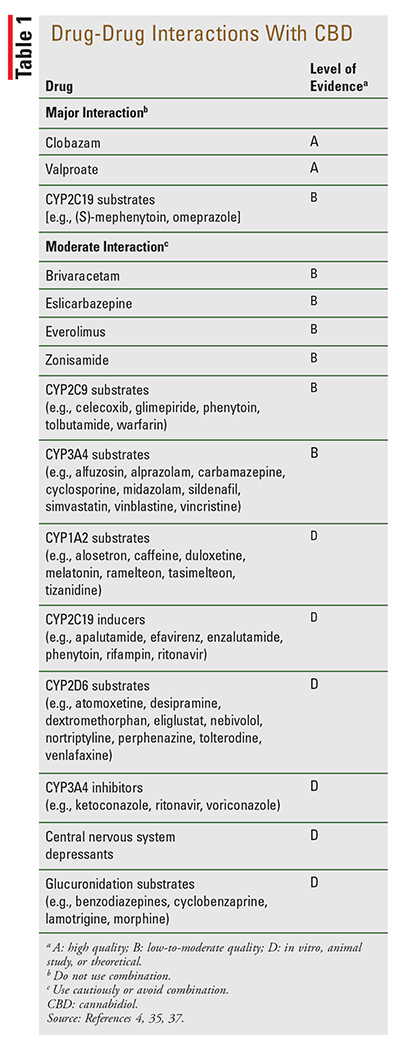
The Pharmacist’s Role
A recent survey by the Arthritis Foundation revealed significant use of and interest in CBD for arthritis. The Foundation acknowledges the possible efficacy of CBD for treating pain, insomnia, and anxiety while also recognizing the lack of rigorous clinical studies.36 Despite a scarcity of evidence for CBD use in the geriatric population, education on known and potential benefits and risks is vital to a patient’s decision-making process. The pervasive direct-to-consumer advertising and ubiquity of CBD products may foster misinformation or misinterpretation of actual evidence. The pharmacist should be prepared to give an unbiased assessment of CBD, including concerns about product mislabeling, underlabeling and overlabeling of CBD, and lack of THC labeling in a product containing it.
The pharmacist should consider patient-specific factors when discussing CBD use. A review of potential drug-drug interactions is warranted prior to using CBD. Counseling on pharmacokinetic variables, such as oral administration with or without food, may be relevant. Comorbidities may also be pertinent to the discussion, and safety concerns should be reinforced. For example, a patient with preexisting respiratory disease should avoid inhalation as the route of CBD administration. An honest and impartial discussion will facilitate a stronger patient–healthcare provider relationship.
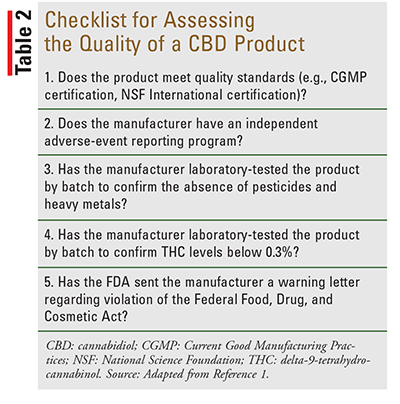
If a patient has decided to use CBD, the pharmacist can direct the patient toward a top-quality CBD product. TABLE 2 provides questions to consider when recommending a CBD product. Given the increasing number of states and U.S. territories legalizing marijuana for medicinal or recreational use, the informed pharmacist will be a valuable resource for discussing the use and safety of CBD with older adults.6
REFERENCES
1. VanDolah HJ, Bauer BA, Mauck KF. Clinicians’ guide to cannabidiol and hemp oils. Mayo Clin Proc. 2019;94(9):1840-1851.
2. Agriculture Improvement Act, S. 10113, 115th Cong. (2018) or S. 12619, 115th Cong. (2018).
3. Brenan M. 14% of Americans say they use CBD products. https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx. Accessed December 29, 2019.
4. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84(11):2477-2482.
5. Bruni N, Della Pepa C, Oliaro-Bosso S, et al. Cannabinoid delivery systems for pain and inflammation treatment. Molecules. 2018;23(10):E2478.
6. Schmitz N, Richert L. Pharmacists and the future of cannabis medicine. J Am Pharm Assoc (2003). 2020;60(1):207-211.
7. Epidiolex (cannabidiol) package insert. Carlsbad, CA: Greenwich Biosciences, Inc; June 2018.
8. FDA. 2019 warning letters and test results. Silver Spring, MD: FDA; 2019.
9. Poklis JL, Mulder HA, Peace MR. The unexpected identification of the cannabimimetic, 5F-ADB, and dextromethorphan in commercially available cannabidiol e-liquids. Forensic Sci Int. 2019;294:e25-e27.
10. FDA. 2015 warning letters and test results. Silver Spring, MD: FDA; 2015.
11. FDA. 2016 warning letters and test results. Silver Spring, MD: FDA; 2016.
12. FDA. 2017 warning letters and test results. Silver Spring, MD: FDA; 2017.
13. FDA. 2018 warning letters and test results. Silver Spring, MD: FDA; 2018.
14. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
15. Consroe P, Sandyk R, Snider SR. Open label evaluation of cannabidiol in dystonic movement disorders. Int J Neurosci. 1986;30(4):277-282.
16. Hurd YL, Spriggs S, Alishayev J, et al. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry. 2019;176(11):911-922.
17. Heussler H, Cohen J, Silove N, et al. A phase 1/2, open-label assessment of the safety, tolerability, and efficacy of transdermal cannabidiol (ZYN002) for the treatment of pediatric fragile X syndrome. J Neurodev Disord. 2019;11(1):16.
18. Morgan CJ, Das RK, Joye A, et al. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav. 2013;38(9):2433-2436.
19. Samara E, Bialer M, Mechoulam R. Pharmacokinetics of cannabidiol in dogs. Drug Metab Dispos. 1988;16(3):469-472.
20. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804.
21. Patrician A, Versic-Bratincevic M, Mijacika T, et al. Examination of a new delivery approach for oral cannabidiol in healthy subjects: a randomized, double-blinded, placebo-controlled pharmacokinetics study. Adv Ther. 2019;36(11):3196-3210.
22. Birnbaum AK, Karanam A, Marino SE, et al. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia. 2019;60(8):1586-1592.
23. Ohlsson A, Lindgren JE, Andersson S, et al. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed Environ Mass Spectrom. 1986;13(2):77-83.
24. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
25. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473.
26. GW Pharmaceuticals. Sativex® commences US Phase II/III clinical trial in cancer pain. www.gwpharm.com/about/news/sativexr-commences-us-phase-iiiii-clinical-trial-cancer-pain. Accessed December 31, 2019.
27. Donvito G, Nass SR, Wilkerson JL, et al. The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology. 2018;43(1):52-79.
28. Gooneratne NS, Vitiello MV. Sleep in older adults: normative changes, sleep disorders, and treatment options. Clin Geriatr Med. 2014;30(3):591-627.
29. Tringale R, Jensen C. Cannabis and insomnia. Depression. 2011;4(12):0-68.
30. Carlini EA, Cunha JM. Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol. 1981;21(suppl 1):S417-S427.
31. Bergamaschi MM, Queiroz RH, Chagas MH, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36(6):1219-1226.
32. Linares IM, Zuardi AW, Pereira LC, et al. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz J Psychiatry. 2019;41(1):9-14.
33. Cannabidiol. In: Natural Medicines Comprehensive Database [subscription required]. Stockton, CA: Therapeutic Research Center. https://naturalmedicines.therapeuticresearch.com. Accessed December 16, 2019.
34. Lloyd SL, Striley CW. Marijuana use among adults 50 years or older in the 21st century. Gerontol Geriatr Med. 2018 Jun 21;1-14.
35. Gaston TE, Bebin EM, Cutter GR, et al. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58(9):1586-1592.
36. Arthritis Foundation. CBD guidance for adults with arthritis. www.arthritis.org/living-with-arthritis/pain-management/chronic-pain/arthritis-foundation-cbd-guidance-for-adults.php. Accessed December 31, 2019.
37. FDA. Drug development and drug interactions: table of substrates, inhibitors and inducers. www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers. Accessed December 31, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.





