US Pharm. 2009;34(2):25-27.
Cardiovascular disease (CVD), the leading cause of death worldwide, involves mechanisms that have yet to be fully understood. While plasma lipids are biologically necessary for cell-membrane synthesis and hormone production and are a source of free fatty acids, dyslipidemia has long been linked to increased incidences of CVD, stroke, and peripheral arterial disease.1
Traditional Risk Stratification
The current treatment approach for dyslipidemia is specified in the National Cholesterol Education Program Adult Treatment Panel (ATP) III.2 In ATP III, risk factors and subsequent risk stratification are derived by calculating what is known as a Framingham risk score (FRS), which has been used for decades to predict CVD risk. ATP III provides recommendations for risk stratification according to multiple risk factors, diabetes, and metabolic syndrome. Risk factors for coronary heart disease (CHD) include hypertension (blood pressure ≥140/90 or use of antihypertensive); family history (first-degree relative with CHD [male, <55 years; female, <65 years]); age (male, ≥45 years; female, ≥55 years); high-density lipoprotein (HDL) level exceeding 40 mg/dL; and smoking. An HDL level exceeding 50 mg/dL is identified as being cardioprotective and is therefore considered a "negative" risk factor. In addition to listing specific CHD risk factors, ATP III identifies CHD risk equivalents (conditions that confer a similar risk for a CHD event). CHD risk equivalents include carotid artery disease, abdominal aortic aneurysm, peripheral arterial disease, diabetes mellitus, or a 10-year risk of myocardial infarction (MI) of at least 20% (i.e., FRS ≥20%).1,2
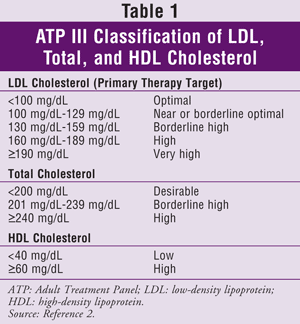
ATP III specifies low-density lipoprotein (LDL) as the primary target for cholesterol treatment. Achieving target triglyceride (TG) and HDL levels is generally considered a secondary goal. Cholesterol goals for the general population are given in TABLE 1.2
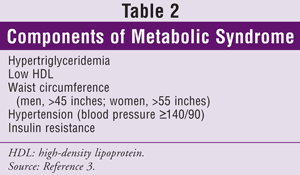
An update to ATP III was published in 2004.3 This version includes a recommendation for a 30% to 40% reduction from baseline LDL in patients considered to be at moderate or high risk for CHD and guidelines under which a patient is considered to be at very high risk. Patients at very high risk may include those with established CVD plus one or more of the following: multiple major risk factors (e.g., diabetes); severe and poorly controlled risk factors (e.g., smoking, uncontrolled hypertension); multiple components of metabolic syndrome (TABLE 2); or acute coronary syndrome.
The ATP III update also suggests that lipid-lowering medication and an LDL goal of less than 100 mg/dL be considered for certain patients.3 These patients should have a 10-year CHD risk of 10% to 20% and an LDL level between 100 mg/dL and 130 mg/dL. They also should be of advanced age and have severe risk factors; TG level 200 mg/dL or greater, non-HDL level 160 mg/dL or greater, and HDL level 40 mg/dL or less; metabolic syndrome; or emerging risk factors such as high-sensitivity C-reactive protein (hs-CRP) (<3 mg/dL) or coronary calcium greater than the 75th percentile for a person's age and sex.
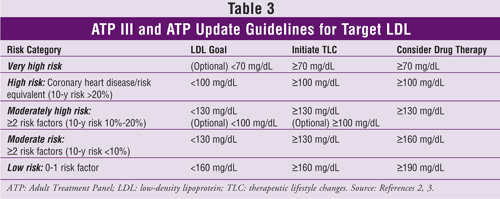
Target LDL levels, recommendations for therapeutic lifestyle changes, and information about initiation of cholesterol-lowering medications (based on the number of CHD risk factors or equivalents) from ATP III and its update are given in TABLE 3.2,3
Nontraditional Risk Markers
Traditional prediction of CVD risk relies heavily on FRS. There are limitations to this approach, however, as FRS has been noted to overestimate risk in low-risk populations and underestimate risk in high-risk populations.4 In fact, sudden death and nonfatal MI with no prior symptoms occur in up to 25% of patients with CHD.5 As previously stated, the ATP III update recommends consideration of more aggressive lipid treatment in patients with emerging risk factors.3 For the past decade, research has focused on the role nontraditional risk factors play in the pathophysiology of CVD. Fibrinogen, hs-CRP, coronary calcium, and lipoprotein subfraction analysis have been the subjects of much research.
Although several markers of inflammation have been associated with CVD, only those that can be measured easily are of practical value.6 One such inflammatory marker is hs-CRP.7 Elevated hs-CRP has been associated with an increased risk of CVD, stroke, and peripheral vascular disease in patients with and without known atherosclerosis. Levels of hs-CRP have been divided into tertiles. These tertiles (<1.0 mg/L, 1 mg/L-3 mg/L, and >3 mg/L) correspond, respectively, with FRS of low, moderate, and high risk.8
The most recent major clinical trial examining the utility of CRP in predicting cardiovascular risk is the JUPITER trial.9 This trial specifically included healthy patients with normal cholesterol (LDL <130 mg/dL) and an elevated CRP level (>2 mg/dL). These patients were treated with rosuvastatin 20 mg or placebo to evaluate the composite endpoint of subsequent MI or nonfatal stroke and the composite risk of MI, stroke, and death from cardiovascular causes. The study was stopped after 1.9 years of follow-up because treatment with rosuvastatin was found to reduce the composite endpoint by 44% compared with placebo. This has been a landmark study because it demonstrates that patients with no cardiovascular risk factors other than elevated CRP obtained a significant benefit from rosuvastatin therapy; however, the cost-effectiveness of screening healthy individuals for elevated CRP and expanding the prescribing of statins in such cases is unknown.
Besides statins, other lipid-lowering agents (such as niacin, fibrates, and ezetimibe), as well as thiazolidinediones and raloxifene, have been shown to reduce CRP levels. It is currently recommended to consider hs-CRP levels in the treatment decision-making process for patients with an FRS-predicted CVD risk of 10% to 20% in the next 10 years, and to avoid routine measurement of hs-CRP in the general adult population.7,8,10
Fibrinogen also has been studied as a nontraditional predictor of CVD risk. In the Coronary Artery Risk Development in Young Adults (CARDIA) study, elevated fibrinogen level in subjects aged 25 to 37 years was associated with an increased risk of subclinical CVD in a patient's subsequent decade of life.10 Fibrinogen plays an important role in platelet aggregation and fibrin formation, both of which are key components of atherosclerosis and thrombus development.1,11 Patients in the CARDIA study who had elevated fibrinogen levels were found to have increased carotid intimal thickness and coronary-artery calcification (CAC) in the subsequent decade. At this time, however, routine measurement of fibrinogen levels is not recommended.10
CAC develops in atherosclerotic coronary arteries and is virtually absent in a normal vessel wall. Higher CAC scores are associated with increased specificity, but decreased sensitivity, for cardiovascular risk prediction. It is important to note, however, that although elevated CAC scores correlate with an increased risk of CVD, cardiovascular events still may occur in a patient with a CAC score of zero. The prognostic value of measuring CAC has been much debated in the past decade. Since 2000, there has been growing evidence that CAC may be independently predictive of cardiovascular outcome over and above the traditional risk factors. In 2007, the American College of Cardiology Foundation/American Heart Association Writing Committee published a consensus document regarding the use of CAC in global cardiovascular risk assessment.12 The consensus of the committee was to discourage unselected CAC screening, as it is of limited clinical value for patients at low risk for CHD (FRS <1%/year). As with hs-CR and fibrinogen, routine measurement of CAC is not recommended; however, CAC scores may be a useful tool for modifying risk prediction and potentially altering therapy for appropriately selected asymptomatic, intermediate-risk patients.
Lipoprotein(a), or Lp(a), is identified in the ATP III guidelines as an emerging CVD risk factor.2 Lipids are transported throughout the body as lipid-and-protein complexes known as lipoproteins. Lipoproteins vary in size and density and are designated accordingly as LDL, HDL, and very-low-density lipoprotein (VLDL). The protein constituents in these complexes are referred to as apolipoproteins. Lp(a), a modified form of LDL, consists of an apoprotein(a), or apo(a), molecule linked to the apolipoprotein B, or apo(b), moiety of the traditional LDL particle. It is similar to LDL in both composition and density. It is believed that since the apo(a) component of Lp(a) is structurally similar to plasminogen, it may compete with plasminogen in the body and thus inhibit fibrinolysis. In addition, Lp(a) is speculated to promote cholesterol accumulation, foam-cell development, and propagation of atherosclerotic plaques.1 Both niacin and estrogen have been shown to effectively lower Lp(a); however, no statin effectively does so.13,14 Elevated levels of Lp(a) have been associated with an increased CVD risk in some, but not all, studied populations.15 Currently, there is no evidence that monitoring or lowering Lp(a) levels is clinically useful, and routine measurement is not recommended.16
Apolipoprotein(b) is the major protein component of not only LDL, but also intermediate-density LDL and VLDL. For cardiovascular risk prediction, measuring apo(b) and non-HDL cholesterol levels has recently been proposed to be superior to measuring LDL cholesterol.17 In a post-hoc analysis of the Treating to New Targets and IDEAL studies, it was found that non-HDL cholesterol (the product of subtracting HDL cholesterol from the total cholesterol measurement) and apo(b) were more closely associated with cardiovascular outcomes than LDL cholesterol.18,19 It is speculated that future guidelines may favor non-HDL cholesterol or apo(b) rather than LDL cholesterol as the primary treatment target; currently, however, LDL remains the primary target of cholesterol management.
Hyperhomocysteinemia has been independently linked to an increased risk of CVD, although it is not as strongly associated as the FRS, and the exact causal mechanism is unknown.20 Defects in folate and B12 pathways may result in elevated homocysteine levels, as do diets deficient in folate or vitamins B6 and B12 and medications that interfere with the metabolism or absorption of these vitamins.1 Renal and hepatic failure also can elevate homocysteine. Hyperhomocysteinemia is believed to be fairly uncommon. The elderly population may be at increased risk for this condition, owing to dietary folate deficiency. Measurement of homocysteine levels should be performed when the patient is in the fasting state, with elevated levels prompting an evaluation for etiology.
Finally, lipoprotein subfraction analysis is another potential tool for further cardiovascular risk stratification. It is known that LDL measurement comprises a spectrum of LDL particles ranging from those that are small and dense to those that are lighter and more buoyant. Small, dense LDL particles are noted to confer a higher cardiovascular risk.21 As LDL increases, small, dense LDL subfractions increase. Likewise, total-HDL measurements consist of a spectrum of particles ranging in size and density. Smaller, denser HDL molecules confer less cardioprotective effect. As HDL levels decrease, there is a marked increase in small HDL particles. Based on these findings, it has been suggested that elevated LDL, elevated small LDL subfractions, low HDL, and low, large HDL subfractions are predictive of CVD. The ATP III guidelines recognize that small LDL particles have been identified as a component of atherogenesis and that HDL subfraction measurements may contribute to CVD risk assessment. According to the guidelines, however, the ability of LDL subfractions to predict CVD independent of other risk factors is not well defined. Furthermore, ATP III points out that the clinical utility of HDL subfraction measurement has not been established. Due to this and to the fact that meaningful and reproducible cutoffs for particle size, density, and number have yet to be established, lipoprotein subfraction measurement is not used in the routine management of hyperlipidemia treatment or in risk stratification. It may be useful, however, as an adjunct to traditional risk stratification to determine treatment aggressiveness in an intermediate-risk patient.
Conclusion
Although ATP III-based treatment guidelines, which rely heavily on FRS, remain the core of treatment recommendations in CVD, nontraditional indicators of CVD are emerging as adjuncts to provide more refined risk stratification and assist in the identification of patients with early subclinical disease who may need earlier and more aggressive treatment.
The precise role of nontraditional CVD risk markers in determining a person's future cardiovascular risk, guidelines for target levels, and specific recommendations as to which populations should have these markers routinely measured have yet to be delineated. None of the nontraditional CVD markers is currently recommended for routine screening; however, in patients for whom the decision to initiate therapy (especially medication) is unclear, measurement of these risk factors may be useful. In such situations, the measurement of nontraditional risk factors in addition to traditional CVD risk-prediction tools may confer a significant benefit.
REFERENCES
1. Talbert RL. Hyperlipidemia. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York, NY: McGraw Hill Medical; 2008:385-407.
2. National Heart Lung and Blood Institute. Third report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). www.nhlbi.nih.gov/guidelines/
3. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227-239.
4. Brindle P, Beswick A, Fahey T, Ebrahim S. The accuracy and impact of risk assessment in the primary prevention of cardiovascular disease: a systematic review. Heart. 2006;92:1752-1759.
5. Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med. 1993;119:1187-1197.
6. de Ferranti SD, Rifai N. C-reactive protein: a nontraditional serum marker of cardiovascular risk. Cardiovasc Pathol. 2007;16:14-21.
7. Koenig W, Sund M, Frölich M, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237-242.
8. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for health-care professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499-511.
9. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195-2207.
10. Committee on Cardiovascular and Metabolic Diseases. Assessing cardiovascular disease risk with nontraditional risk factors: the latest evidence. CMDManagement Insights Informat Cardiovasc Metabol Dis. 2008;13(2):1-3,6.
11. Mora S, Rifai N, Buring JE, Ridker PM. Additive value of immunoassay-measured fibrinogen and high-sensitivity C-reactive protein levels for predicting incident cardiovascular events. Circulation. 2006;114:381-387.
12. Greenland B, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 Clinical Expert Consensus Document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain. A report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography). J Am Coll Cardiol. 2007;49:378-402.
13. Angelin B. Therapy for lowering lipoprotein (a) levels. Curr Opin Lipidol. 1997;8:337-341.
14. Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583-1592.
15. Moliterno D, Jokinen EV, Miserez AR, et al. No association between plasma lipoprotein(a) concentrations and the presence or absence of coronary atherosclerosis in African-Americans. Arterioscler Thromb Vasc Biol. 1995;15:850-855.
16. Bennet A, Di Angelantonio E, Erqou S, et al. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch Intern Med. 2008;168:598-608.
17. Kastelein JJ, van der Steeg WA, Holme I, et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation. 2008;117:3002-3009.
18. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425-1435.
19. Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437-2445.
20. Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202-1206.
21. Rizzo M, Berneis K. Low-density lipoprotein size and cardiovascular risk assessment. QJM.
2006;99:1-14.
To comment on this article, contact rdavidson@jobson.com.





