US Pharm. 2021;45(3):6-12.
Approximately one in seven people are estimated to have chronic kidney disease (CKD), but most are unaware they have this condition. Prevalence of CKD increases with age, from 7% of people aged 18 to 44 years to 38% of those aged 65 years or older. Nationally, it is the eighth leading cause of death. A healthy kidney’s function is to remove wastes and toxins from the body, as well as to stimulate red blood cell production, control blood pressure, maintain proper bone health, and regulate essential blood chemicals. CKD is a condition in which the kidneys are damaged and do not filter properly. Due to this diminished kidney function, fluid and waste from the blood remains in the body and can lead to detrimental health problems.1,2
Definition and Classification
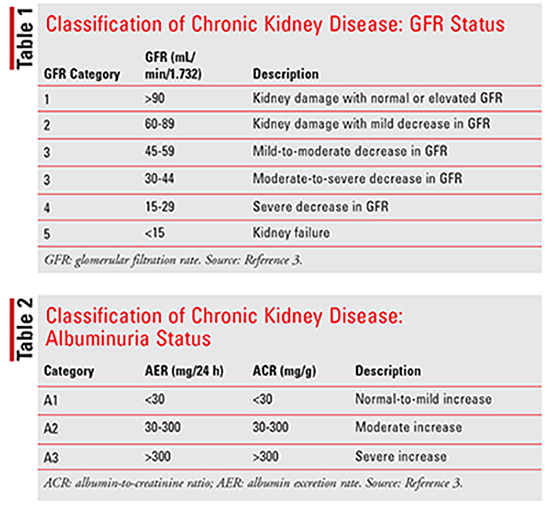
Practice guidelines published by the Kidney Disease: Improving Global Outcomes (KDIGO) organization and the National Kidney Foundation define CKD as abnormalities of kidney structure or function that have been present for at least 3 months with negative implications for a patient’s health. Abnormalities of kidney structure, or kidney damage, are defined by one or more of the following: albuminuria, urine-sediment abnormalities, electrolyte and other abnormalities due to tubular disorders, abnormalities detected by histology, structural abnormalities detected by imaging, and history of kidney transplantation. The rate at which blood is filtered by the glomeruli, or glomerular filtration rate (GFR), is considered to be the best indicator of kidney function; it is generally reduced after widespread structural damage, and most other kidney functions tend to decline in parallel. A GFR less than 60 mL/min/1.73 m2 has been defined as the diagnostic threshold to indicate CKD. Most laboratories now report estimated GFR (eGFR) based on filtration markers, most commonly creatinine.3
CKD can be classified according to GFR (Table 1), albuminuria status (Table 2), and cause of CKD. Evidence has suggested that both decreased GFR and increased levels of albuminuria are independently related to mortality, cardiovascular events, and the rate of progression to end-stage renal disease (ESRD). It is important to recognize that GFR alone may not be enough to identify stage 1 and 2 CKD because GFR can be normal or near normal. In these patients, the presence of kidney damage, as previously described, can help determine the diagnosis. The cause of CKD is usually defined by the presence of systemic disease, such as diabetes, atherosclerosis, malignancy, autoimmune disorders, and chronic infections, and the location of anatomic abnormalities, which include glomerular, tubulointerstitial, vascular, and cystic/congenital diseases.3,4
Causes and Risk Factors
Diabetes and hypertension are two of the most common causes of CKD; obesity is another major contributor. Nearly 50% of diabetic adults have CKD; the prevalence of CKD among those with hypertension is about 30%, and it is 17% in patients who are obese. Aging is also a consideration. GFR declines with age in the absence of kidney disease beginning at age 30 to 40 years, with an increased rate of decline after ages 65 to 70 years. In addition, renal blood flow progressively decreases with aging. These changes may predispose an older kidney to the development of CKD. Other factors such as nephrotoxin exposure, kidney stones, fetal and maternal factors, infections, environmental factors, and acute kidney injury also play a role in the development of CKD.5-7
Clinical Presentation
Many people will not know they have CKD because most will be asymptomatic; symptom development is typically uncommon with a GFR greater than 35 mL/min/1.73 m2. Less commonly, patients may pre-sent with gross hematuria, foamy urine, nocturia, flank pain, or decreased urine output. Identification of CKD is usually made following findings from screening tests, which is why screening tests may play an important role in early detection. The U.S. Preventive Services Task Force does not currently recommend routine screening in asymptomatic adults, and similarly, the American College of Physcians recommends against screening for CKD in asymptomatic patients without risk factors. However, the American Society of Nephrology strongly recommends regular screening for kidney disease regardless of risk factors. Other professional organizations such as the American Diabetes Association and the National Kidney Foundation support screening for CKD in at-risk patients. Screenings can be performed at routine check-ups with serum chemistry profiles and urine studies, evaluating serum creatinine for estimating GFR, and checking for albuminuria quantified by a urine albumin-to-creatinine ratio. Early detection may help slow or prevent the progression to ESRD.8-10
Management
The management of CKD focuses on delaying or preventing its progression. A major focus is on addressing cardiovascular health, which may directly and indirectly impact CKD progression. A majority of patients with CKD have cardiovascular disease (CVD) compared with those without CKD. Cardiovascular complications are the leading cause of mortality among patients with ESRD. Therefore, a major component of CKD management is reduction of cardiovascular risk. This can be achieved by blood pressure control, interrupting the renin-angiotensin-aldosterone system (RAAS), and controlling blood sugar and dyslipidemia.3
High blood pressure and CKD constitute a self-reinforcing cycle. Hypertension may occur as a result of kidney disease, but hypertension may also accelerate further kidney damage. It is imperative to lower blood pressure to help prevent further kidney damage and functional decline. Hypertension is also an important risk factor for CVD, increasing patients’ risk for cardiovascular and cerebrovascular events, especially when proteinuria is present. The KDIGO and the Eighth Joint National Committee (JNC 8) guidelines recommend a blood pressure goal of less than 140/90 mm Hg for adults with CKD. In addition, the KDIGO further states that patients with urine albumin excretion of 30 mg/24 hours or more should maintain a blood pressure of 130/80 mm Hg or less. The American College of Cardiology/American Heart Association (ACC/AHA) Hypertension Guideline recommends that all patients with CKD maintain a blood pressure of less than 130/80 mm Hg. To help patients achieve lower blood pressures, the guidelines recommend lifestyle and pharmacologic therapies.
Patients should be encouraged to incorporate lifestyle modifications such as maintaining a healthy weight with a BMI between 25 and 30, lowering salt intake to less than 2 g per day, participating in routine exercise with a goal of at least 30 minutes a day five times per week, and limiting alcohol intake to no more than two standard drinks per day for men and no more than one standard drink per day for women. Smoking cessation should also be encouraged.3,4,11-13
In addition to lifestyle modifications, many patients will require pharmacologic therapy to help reduce their blood pressure. There are multiple guidelines to help determine which agents should be used, including JNC 8 and the 2017 ACC/AHA Hypertension Guideline. Most patients will require two or more antihypertensive agents to achieve their target blood pressure. With the exception of CKD patients with high levels of urinary protein, there is no strong evidence to support the use of any one antihypertensive agent in the management of hypertension in CKD patients.
Blockade of the RAAS with either an angiotensin-converting enzyme inhibitor (ACEI) or an angiotensin II receptor blocker (ARB) has been shown to be beneficial in patients with diabetic nephropathy and in nondiabetic CKD patients with proteinuria. The presence of excess protein in the urine is indicative of kidney damage; the degree of proteinuria generally predicts the rate of CKD progression. Data from clinical trials have shown ACEIs and ARBs to be renal-protective, having beneficial effects on kidney function. These agents are considered to be first-line therapy for hypertension treatment in diabetic CKD patients and in CKD patients who are nondiabetic with proteinuria. It is recommended to initiate an ACEI or ARB for adults with diabetes whose urinary albumin excretion is 30 mg to 300 mg/24 hours and for all patients with and without diabetes whose urinary albumin excretion is more than 300 mg/24 hours. These agents are safe to combine with most other antihypertensive agents. It is important to recognize, however, that these agents can cause significant increases in potassium levels and that a temporary reduction in GFR may occur. Combination therapy with an ACEI and ARB for the management of proteinuria is not recommended due to the increased risk of hyperkalemia and acute kidney injury.3,11,14,15
Diabetes is the leading cause of CKD and ESRD; therefore, it is important to optimally manage diabetic patients to help delay progression of CKD. In the most recent KDIGO Diabetes Management in CKD guideline, target hemoglobin A1C goals should be individualized based on patient factors, such as comorbidities, life expectancy, and risk for hypoglycemia. This approach is consistent with other guidelines from leading diabetes organizations worldwide. Hemoglobin A1C goals may vary from less than 6.5% to less than 8%. The treatment algorithm for antihyperglycemic drugs for patients with type 2 diabetes (T2D) and CKD should include lifestyle modifications, a first-line therapy of metformin and a sodium–glucose cotransporter-2 (SGLT-2) inhibitor, and any additional drug therapy needed for optimal glycemic control. Metformin may help patients achieve weight loss and reduce the risk for cardiovascular events. The SGLT-2 inhibitors have been shown to be cardioprotective and renoprotective, significantly reducing the risk of cardiovascular events and delaying the progression of CKD in diabetics. Patients should be started on metformin only if their eGFR is 45 mL/min/1.73 m2 or greater and an SGLT-2 inhibitor if their eGFR is 30 mL/min/1.73 m2 or greater.
The glucagon-like peptide receptor agonists (GLP-1 RAs) may be another class of medications to consider. In addition to improving glucose control, they lower blood pressure and confer weight loss. These agents also reduce cardiovascular events and have been shown to provide favorable kidney benefits, reducing albuminuria and preserving eGFR. The GLP-1 RAs may be considered as add-on therapy in patients with T2D currently on metformin and an SGLT-2 inhibitor who have not achieved individualized glycemic targets or in patients who cannot tolerate those medications.4,16
Dyslipidemia is a contributing factor for the development of cardiovascular disease in CKD patients. Instead of using LDL-C levels to guide the management of dyslipidemia, the KDIGO guidelines recommend that all CKD patients aged 50 years and older, regardless of GFR category, be started on a statin or a statin/ezetimibe combination. Patients who are aged 50 years or older with CKD have a 10-year coronary heart disease risk greater than 10%, suggesting that they are a high-risk population. In CKD patients younger than age 50 years, statin treatment is recommended in those with known coronary artery disease, diabetes, prior ischemic stroke, or in those who have more than a 10% estimated 10-year incidence of coronary death or nonfatal myocardial infarction.17,18
Complications of CKD
Progressive CKD is associated with the development of several complications that contribute to higher morbidity and mortality and lower quality of life for patients. Some of these complications include the risk of developing anemia, electrolyte abnormalities, and mineral and bone disorders. Frequent monitoring and assessment of laboratory abnormalities are crucial to help identify the development of such complications.
Anemia is probably the most common complication of CKD, mostly seen in stages 3 to 5 CKD. When the kidneys are damaged, less erythropoietin, an essential hormone that signals the bone marrow to produce red blood cells, is produced. Treatment usually includes a combination of iron supplementation and erythropoiesis-stimulating agents (ESAs). The use of ESAs, however, has been associated with an increased risk of death, stroke, and venous thromboembolism; balancing the potential benefits of reducing blood transfusions and anemia-related symptoms must be weighed against the risk of harm in individual patients.3,19,20
Electrolyte abnormalities are another common complication seen in patients with progressive CKD. Initial treatment strategies include dietary restrictions and use of dietary supplements. Hyperkalemia is one of the most common and life-threatening electrolyte disorders in CKD, causing cardiac toxicity and life-threatening arrythmias. Dietary potassium restriction is the mainstay of treatment. If dietary measures alone are not enough, the use of loop or thiazide diuretics can be initiated to increase urinary potassium excretion. Other agents include sodium polystyrene sulfonate, patiromer, and sodium zirconium cyclosilicate. Declining kidney function and reduced capacity of the damaged kidneys to synthesize ammonia and excrete hydrogen ions put the CKD patient at an increased risk for metabolic acidosis. Serum bicarbonate levels should be kept at levels between 22-32 mmol/L to help minimize this complication. Levels less than 22 mmol/L have been associated with increased risk of CKD progression and increased risk of death. To help maintain serum bicarbonate levels in patients who are persistently below 22 mmol/L, oral bicarbonate supplementation should be given.4,21
Kidneys help regulate serum phosphate and calcium levels. As kidney function declines, abnormalities in serum calcium and phosphate become more prominent, making mineral and bone disorders another common complication in CKD. Increased serum phosphate leads to a decrease in serum calcium. Additionally, CKD impairs the kidney’s ability to convert vitamin D to calcitriol. Management usually requires a combination of dietary interventions, phosphate-binding medications, and vitamin D supplementation to maintain normalized levels of parathyroid hormone, phosphorus, and calcium.4,22
Role of the Pharmacist
The successful care of CKD patients requires an interdisciplinary healthcare team. Pharmacists can contribute greatly to this team and are ideally situated to help manage patients with CKD. These patients have multiple comorbidities and are taking multiple medications, therefore there is greater potential for medication-related problems. Pharmacists can identify such problems by evaluating the patient medical record for drug interactions, laboratory monitoring, and determining the need for dose adjustments. More importantly, pharmacists can assist patients with their drug therapies; many patients may have high pill burdens, creating complex medication regimens. Nonadherence can range up to 74% in CKD patients, which can have detrimental effects leading to the progression of CKD. Improving patient education and understanding of CKD are important factors in increasing patient adherence.23,24
It is also important for patients with CKD to avoid nephrotoxins, which can include medications. There are a number of medications that patients should avoid, and the pharmacist can aid in the identification of such agents. Routine use of nonsteroidal anti-inflammatory agents should be avoided, especially in patients who are taking ACEIs or ARBs. Patients should be discouraged from using herbal supplements, because many of these products are known to cause nephrotoxicity and may contain minerals such as potassium that can be harmful to CKD patients. In addition, many herbs potentially interact with a myriad of medications, putting the patient at an increased risk of experiencing a serious reaction.25-27
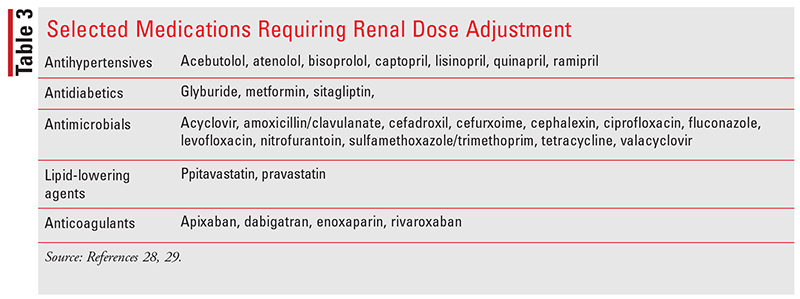
Many medications are eliminated by the kidneys, and adjustments in medication dosing are often needed in CKD patients (Table 3). Alternatively, many medications have toxic metabolites that accumulate in the presence of decreased renal function. Pharmacists have the training to review renal function and determine appropriate doses or provide recommendations for alternate therapy that may minimize the risk of adverse drug events.
Conclusion
CKD is a public health concern, with almost 15% of the U.S. population affected. It is associated with high morbidity and mortality and is a leading cause of death. Management of CKD focuses on reducing a patient’s cardiovascular risk and decreasing proteinuria through lifestyle modifications and achieving optimal blood pressure and glycemic control. If progression of CKD is not slowed or prevented, it can have many consequences. Patients need to be monitored for complications such as anemia, electrolyte imbalances, and related bone mineral abnormalities. CKD patients usually have high medication burdens. This degree of polypharmacy along with the comorbid conditions that arise in an individual increases risk for medication-related problems. Through multidisciplinary team management of CKD patients, pharmacists play a pivotal role in identifying such medication-related problems and increasing patient knowledge about the disease state as well as its associated therapies.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.
Why are the kidneys so important?
The kidneys’ main roles are to remove waste products and extra water from your body, help control blood pressure, help make red blood cells, and help keep bones healthy. The kidneys dispose of waste products and turn them into urine.
What causes CKD?
Diabetes and high blood pressure are the most common causes of chronic kidney disease (CKD). Other causes include taking medications that can harm the kidneys, kidney stones, genetic factors, infections, environmental factors, and acute kidney injury.
How do I know if I have CKD?
Many people will not know they have CKD because there are usually no symptoms, especially with early disease. As the disease gets worse, you may have swelling in your feet, ankles, or legs. You might feel very tired, have high blood pressure, and have decreased urine output. Getting tested may be the only way you know you have kidney disease. If you have high blood pressure or diabetes, it is very important to discuss getting screened for kidney disease with your healthcare provider.
Can I prevent CKD?
Yes. You should see your healthcare provider regularly. If you have high blood pressure, be sure to control it. If you have diabetes, you should control your blood sugar levels. Lose weight if you are overweight or obese. Try to exercise at least 30 minutes a day five times per week, and limit alcohol intake to no more than two standard drinks per day for men and no more than one standard drink per day for women. Try to stop smoking if you are currently a smoker.
How is CKD treated?
You and your healthcare provider will determine what the best plan will be for you. But treatments will include lifestyle changes and the use of medicines to help slow or prevent the progression of the disease. It is very important to continue to take your medicines every day. If they cause side effects or are too expensive, speak with your healthcare provider or pharmacist about it. They will be able to help you with other options. It is also very important to talk with your pharmacist before taking any OTC medications or supplements. Many of these products can worsen your condition.
What if my kidneys stop working?
Kidney failure is when your kidneys stop working well enough to keep you alive. There is no cure, but there are options that you and your healthcare provider can talk about. These include having kidney-transplant surgery, having your blood filtered by a machine at least three times a week (hemodialysis), or using a special fluid that is piped in and out of your belly (peritoneal dialysis).
Where can I go for more information?
Visit the CDC at www.cdc.gov/kidneydisease/basics.html or the National Institute of Diabetes and Digestive and Kidney Diseases at www.niddk.nih.gov/health-information/kidney-disease/chronic-kidney-disease-ckd, or call the National Kidney Foundation Care Patient Help Line at 855-NKFCARES (855-653-2273), or visit www.kidney.org.
REFERENCES
1. CDC. Chronic kidney disease in the United States, 2019. Published March 13, 2019. www.cdc.gov/kidneydisease/publications-resources/2019-national-facts.html. Accessed February 10, 2021.
2. Kochanek KD, Xu J, Arias E. Mortality in the United States, 2019. NCHS Data Brief. 2020;(395):1-8.
3. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
4. Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713-735.
5. Luyckx VA, Tuttle KR, Garcia-Garcia G, et al. Reducing major risk factors for chronic kidney disease. Kidney Int Suppl. 2017;7(2):71-87.
6. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238-1252.
7. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419-428.
8. Final recommendation statement: chronic kidney disease: screening. United States Preventive Services Task Force. www.uspreventiveservicestaskforce.org/uspstf/document/RecommendationStatementFinal/chronic-kidney-disease-ckd-screening. Accessed February 11, 2021.
9. Qaseem A, Hopkins RH Jr, Sweet DE, et al. Clinical Guidelines Committee of the American College of Physicians. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159(12):835-847.
10. Berns JS. Routine screening for CKD should be done in asymptomatic adults … selectively. Clin J Am Soc Nephrol. 2014;9(11):1988-1992.
11. Taler SJ, Agarwal R, Bakris GL, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis. 2013;62(2):201-213.
12. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College Of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115.
13. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
14. Mann JFE, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372(9638):547-553.
15. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;(2):337-414.
16. de Boer IH, Caramori ML, Chan JCN, et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98(4):839-848.
17. Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group. KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int Suppl. 2013;3:259-305.
18. Sarnak MJ, Bloom R, Muntner P, et al. KDOQI US commentary on the 2013 KDIGO clinical practice guideline for lipid management in CKD. Am J Kidney Dis. 2015;65(3):354-366.
19. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2(4):279-335.
20. Kliger AS, Foley RN, Goldfarb DS, et al. KDOQI US commentary on the 2012 KDIGO Clinical Practice Guideline for Anemia in CKD. Am J Kidney Dis. 2013;62(5):849-859.
21. Dhondup T, Qian Q. Electrolyte and acid-base disorders in chronic kidney disease and end-stage kidney failure. Blood Purif. 2017;43(1-3):179-188.
22. Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO chronic kidney disease–mineral and bone disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int. 2017;92(1):26-36.
23. Moreira LB, Fernandes PFCBC, Mota RS, et al. Medication noncompliance in chronic kidney disease. J Nephrol. 2008;21(3):354-362.
24. Mechta Nielsen T, Frøjk Juhl M, Feldt-Rasmussen B, Thomsen T. Adherence to medication in patients with chronic kidney disease: a systematic review of qualitative research. Clin Kidney J. 2018;11(4):513-527.
25. Naughton CA. Drug-induced nephrotoxicity. Am Fam Physician. 2008;78(6):743-750.
26. Yang Y-X, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953.
27. National Kidney Foundation. Use of herbal supplements in chronic kidney disease. https://kidneyhi.org/use-of-herbal-supplements-in-chronic-kidney-disease. Accessed February 14, 2021.
28. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
29. Hassan Y, Al-Ramahi R, Abd Aziz N, Ghazali R. Drug use and dosing in chronic kidney disease. Ann Acad Med Singap. 2009;38(12):1095-1103.
To comment on this article, contact rdavidson@uspharmacist.com.