US Pharm. 2012;37(5):HS-9-HS-12.
Cancer pain affects one in three patients and significantly impacts quality of life.1 The incidence of oncologic pain ranges from 25% to 75%, depending upon the extent of cancer progression.2 While adequate pain relief is achievable, there are many barriers to effective pain control that may be attributed to providers, patients, or systematic problems (e.g., drug shortages or financial barriers). Inadequate pain evaluation, addiction concerns, financial burden, and adverse effects are some barriers.3 Utilizing tools to properly assess the quality (i.e., visceral, somatic, or neuropathic) and severity of the pain is crucial for attaining pain control. Identifying obstacles is critical for the alleviation of cancer pain. This review will discuss the pharmacologic opioid and nonopioid treatment of cancer pain.
Opioid Therapies
Once the psychological, social, and behavioral aspects of the patient’s pain have been distinguished, the practitioner can concentrate on pharmacologic therapies for pain control. Current practice is heavily guided by observational experience, in part because of limited evidence validating the appropriateness of existing guidelines for treatment.4 The analgesic ladder developed by the World Health Organization (WHO) is a guideline that involves the use of opioids as a principal component of cancer pain pharmacotherapy and uses a stepwise approach based on pain severity.5 Generally, approaches to pain control rely on regular monitoring and pain assessment using patient self-report scales to guide medication adjustments.6 The most popular and prevalent pain rating scales are the visual analogue, numerical, and verbal scales.6
Opioids are the mainstay of treatment for severe pain, although a broad selection of nonopioid analgesics, such as acetaminophen (APAP) and nonsteroidal anti-inflammatory drugs (NSAIDs), is also available.6 Opioid analgesia affects the mu and kappa receptors, the primary receptors responsible for pain transmission. Although no opioid is pharmacologically superior to another in terms of drug initiation, morphine and codeine are frequently selected first, presumably because of their prevalence and practitioners’ familiarity with dosing.7 Pure mu agonist opioids include codeine, hydrocodone, morphine, hydromorphone, oxycodone, oxymorphone, levorphanol, methadone, and fentanyl.7 Opioids are usually reserved for moderate-to-severe pain when acetaminophen or NSAIDs have been unsuccessful in alleviating the pain.
Opioid Formulations
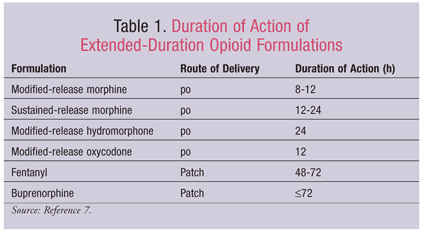
Tumor-related pain is typically chronic and unmitigating irrespective of cancer stage and necessitates long-lasting, round-the-clock coverage.4 Drugs with long half-lives are preferred for maintenance therapy to eliminate the need for frequent dosing and to minimize possible breakthrough pain. The strategy for control of tumor pain involves two tiers: the first tier supplies baseline control and the second tier controls periods of breakthrough pain. Immediate-release and extended-release formulations have been developed that contain the ideal pharmacodynamic properties to deliver both types of control. Baseline pain control creates a niche for extended-release formulations, which supply continuous analgesic coverage.7 Extended-release formulations include modified-release morphine, sustained-release morphine, modified-release hydromorphone, and modified-release oxycodone (TABLE 1). Breakthrough pain is ideally treated with fast-acting, rapidly absorbed drugs with a short duration of action, attributes that are consistent with formulations such as transmucosal fentanyl. The goal is to individualize drug combinations to maximize the patient’s pain-free periods and minimize adverse effects, thereby allowing normal daily functioning.
Routes of Opioid Administration
For patients who are able to swallow, oral routes are preferred to parenteral routes to avoid complications such as injection-site discomfort, difficulty of administration, and infection. Transdermal administration (via fentanyl and buprenorphine patches) is a suitable route for patients who find compliance difficult or who cannot tolerate morphine.6 Fentanyl and methadone are appropriate opioids for patients with reduced renal function, as these drugs are metabolized primarily by the liver.8 Intravenous (IV), intramuscular (IM), and subcutaneous (SQ) injections are alternative drug-delivery methods that may be considered in patients with advanced disease who require high opioid doses. Parenteral routes are available for morphine, hydromorphone, oxymorphone, levorphanol, fentanyl, and methadone, although IM injections typically are avoided because of unappreciable pharmacokinetic advantage and increased injection-site pain relative to IV and SQ routes.6 The IV dose of opioids is approximately 33% of the oral dose.6
Owing to interpatient variability and an erratic pharmacodynamic profile, methadone is best administered by an experienced practitioner in order to prevent accidental overdose or inadequate pain-relief titration.6 Buprenorphine (a partial mu receptor agonist and kappa receptor antagonist) and tramadol (a centrally acting drug with mixed mechanisms) are other analgesic options. Tramadol should not be used concomitantly with monoamine oxidase inhibitors, and its use in patients with a history of seizures or concomitant antidepressants should be carefully evaluated prior to initiation because of the increased risk of seizure or serotonin syndrome.8
Opioid Titration
Dose titration is crucial for optimizing and individualizing pain control. Incremental increases can be based on 30% to 100% of the total daily dose or on the daily dose of breakthrough analgesia required.6,7 Excessive dose escalation, which may overestimate the patient’s analgesic needs, poses the risk of adverse effects, respiratory depression, or death. Rapid achievement of adequate pain control should not outweigh safety, especially with opioids, which are potentially lethal. Doses equivalent to 200 mg of morphine per day should be regarded as outliers.7 Practitioners should address the practicality of taking an excessive number of tablets or determine whether a disproportionate increase in toxic effects is offset by pain control at maximal doses.7
Dose-titration intervals differ among opioids based on time to steady-state concentrations and absorption rates.7 Fast onset and elimination after IV administration allow for more rapid titration and are ideal when dose escalation is preferred for severe pain. Sustained-release formulations may be uptitrated every 2 to 3 days, whereas patches usually require 3 to 6 days and thus are best reserved for stable opioid regimens.6,7 Methadone has variable absorption and elimination characteristics, so more conservative weekly dose escalation is desirable to ensure safety.7 Breakthrough analgesic doses should be titrated to match the patient’s needs and are usually maintained at 5% to 15% of the daily dose of long-acting opioids.7 The avoidance of opioids with similar pharmacokinetic properties (e.g., extended-release forms) in the same medication regimen is recommended.8 A drug may be tapered weekly by 30% to 50% of the cumulative dose when indicated, such as in the case of abating pain; it should never be abruptly halted, as this may precipitate pain recurrence or withdrawal symptoms.8
Rotation of opioid agents is appropriate for patients who experience adverse effects with high doses or who have an inadequate response to rapid titration (i.e., opioid resistance).8 Neuropathic pain, breakthrough pain, and addiction may be more effectively controlled using a two-step process of opioid rotation.9 The first step is to calculate an equianalgesic dose while factoring in a dose reduction of 25% to 50% (except for methadone or fentanyl, which may require reductions of up to 90%) to account for incomplete cross-tolerance and interdependent variation.9 The second step is to adjust the dose based upon pain severity, patient tolerability, worsening disease, and other patient factors that may impact response to analgesia.9
Management of Opioid Adverse Effects
A significant drawback to treating tumor-related pain with opioids is the occurrence of adverse effects, including constipation, delirium, nausea, pruritus, sedation, and respiratory depression.10 It is imperative to anticipate and treat these effects, as quality of life may be largely impacted otherwise.
Constipation, which occurs in more than 50% of patients receiving opioids, is due to the inhibition of intestinal secretions and peristalsis.11 According to the Rome III criteria, constipation is defined as the presence of two or more of the following: straining, hard stools, and a feeling of incomplete evacuation for at least 25% of defecations. Additional criteria are insufficient evidence of irritable bowel syndrome and loose stools occurring only with use of laxatives.12 Although tolerance to opioid-induced adverse effects often develops, this is not the case with constipation; thus, it is advisable to start prophylactic therapy for constipation when opioid therapy is initiated or increased, rather than waiting for onset.10,11,13
The National Comprehensive Cancer Network (NCCN) recommends a goal of one bowel movement every 1 to 2 days. Available agents include stool softeners, laxatives, stimulants, and opioid antagonists. Various combinations are used, as data supporting any one regimen are insufficient; however, in one study, sennosides were more effective as monotherapy than when used concomitantly with docusate.14 Additionally, while an increase in dietary fiber is encouraged, the use of fiber products is not, since these products are unlikely to be effective.10 Opioid antagonists (e.g., almivopan) and motility agents (e.g., metoclopramide) may be used, but generally not as first-line therapy. A Cochrane review of the effects of mu receptor antagonists showed a benefit of almivopan and methylnaltrexone in combating opioid-induced constipation, with an adverse-effect profile comparable to that of placebo.15
Opioid-induced sedation has been reported to occur in 23% to 28% of patients. Although the mechanism has not been fully elucidated, interruptions in REM sleep and inhibition of sensory input may contribute.16 Tolerance to opioids typically develops within 2 to 3 days, assuming a constant dose.16 Per NCCN guidelines, if sedation persists for more than a week, a change in opioid therapy, frequency, or dose should be considered. If this is ineffective, some evidence supports the use of stimulants such as methylphenidate, modafinil, or dextroamphetamine, especially if the sedation limits activities of daily living.17
Nausea in cancer patients is reported to be as high as 60%. To best treat this symptom, it is important to identify the etiology, if possible, since opioids are not always the cause.13 Hypercalcemia, constipation, anxiety, uremia, infection, and chemotherapy, among others, are possible causes.10,13 When opioid-induced nausea is suspected, consider prochlorperazine, thiethylperazine, or haloperidol; metoclopramide may be a better option if the nausea is due to an opioid-induced reduction in gastric motility.10,18 Other options include serotonin receptor antagonists and benzodiazepines, which can be administered according to a schedule for up to a week and then switched to an as-needed basis, if possible.10 Neuroablative and psychological techniques are nonpharmacologic options to reduce the need for opioid use.10
Pruritus attributed to opioids occurs in up to 50% of patients receiving IV formulations and can greatly impact willingness to continue treatment. Antihistamines such as diphenhydramine and promethazine are treatment options, but they may exacerbate the sedative effects of opioids. Naloxone infusion does not carry this risk, and studies have shown it to be effective in opioid-induced pruritus.19
Adjuvant Analgesics for Neuropathic Pain
Given the limited literature on the treatment of neuropathic pain in this specific population, many of the recommendations have been extrapolated from noncancer pain groups.10 Antidepressants and anticonvulsants are mainstays of therapy, according to the NCCN. A recent study showed that pregabalin reduced morphine rescue use compared with amitriptyline, gabapentin, and placebo.20 Gabapentin and pregabalin have been recommended by some sources as first-line therapy for patients with an inadequate response to opioids.7 The Neuropathic Pain Special Interest Group supports the use of tramadol and opioids as first-line agents for cancer-related neuropathic pain.21 Other agents that have been used are oxcarbazepine, lamotrigine, topiramate, mexiletine, lidocaine, baclofen, corticosteroids, and clonazepam.7
NSAIDs and APAP
NSAIDs and APAP, although milder analgesics, have a role in cancer pain management. The WHO’s analgesic pain ladder recommends nonopioids as the first of three escalating steps in managing pain.5 These agents are generally reserved for mild-to-moderate cancer pain, since they exhibit a ceiling effect in terms of analgesic properties.7 One systematic review found no differences within the NSAID class, and the literature has mixed results regarding use in combination with opioids.22 Although NSAIDs may obviate the need for opioids, they must be used with caution in light of the associated gastrointestinal, renal, and cardiovascular toxicities.7 APAP, another effective nonopioid option, is often used in combination with opioids. One study found that the addition of APAP to opioids improved pain relief in cancer patients without causing additional adverse effects when administered for 48 hours.23 In an effort to reduce hepatic risk, restrictions imposed by the FDA in 2011 limit the amount of APAP in products to a maximum of 325 mg per dosage unit.24
Conclusion
Given the right tools and education, practitioners can manage cancer pain effectively. Pharmacists’ interaction with patients, as well as their knowledge and training, positions them well to monitor for critical drug interactions and signs of adverse effects. Additionally, pharmacists are essential in the education and counseling of patients, which is critical for their understanding of their treatment. Although various factors (e.g., patient variability, drug cost, drug tolerability) prevent the recommendation of one particular regimen, trial and error can identify the most effective course of therapy on an individualized basis, and quality of life can be improved. Although cancer pain can be debilitating, appropriately prescribed pharmacologic therapy plays an important role in easing this burden while minimizing adverse effects.
REFERENCES
1. Mayo Clinic. Cancer pain: relief is possible. www.mayoclinic.com/health/cancer-pain/CA00021. Accessed November 30, 2011.
2. Paice JA, Ferrell B. The management of cancer pain. CA Cancer J Clin. 2011;61:157-182.
3. National Cancer Institute. Pain (PDQ®).
www.cancer.gov/cancertopics/pdq/supportivecare/pain/HealthProfessional/page1#top.
Accessed December 1, 2011.
4. Azevedo São Leão Ferreira K, Kimura M, Jacobsen Teixeira M. The
WHO analgesic ladder for cancer pain control, twenty years of use. How
much pain relief does one get from using it? Support Care Cancer. 2006;14:1086-1093.
5. World Health Organization. Cancer Pain Relief: With a Guide to Opioid Availability. 2nd ed. Geneva, Switzerland: World Health Organization; 1996.
6. Jost L, Roila F, ESMO Guidelines Working Group. Management of cancer pain: ESMO clinical recommendations. Ann Oncol. 2009;20(suppl 4):170-173.
7. Portenoy RK. Treatment of cancer pain. Lancet. 2011;377:2236-2247.
8. Krakowski I, Theobald S, Balp L, et al. Summary version of the
Standards, Options and Recommendations for the use of analgesia for the
treatment of nociceptive pain in adults with cancer (update 2002). Br J Cancer. 2003;89(suppl 1):S67-S72.
9. Fine PG, Portenoy RK, Ad Hoc Expert Panel on Evidence Review and
Guidelines for Opioid Rotation. Establishing “best practices” for opioid
rotation: conclusions of an expert panel. J Pain Symptom Manage. 2009;38:418-425.
10. National Comprehensive Cancer Network. NCCN Clinical Practice
Guidelines in Oncology. Adult cancer pain. Version 2.2011.
www.nccn.org/professionals/physician_gls/pdf/pain.pdf. Accessed December
1, 2011.
11. DeVita DT Jr, Lawrence TS, Rosenberg SA, et al, eds. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
12. Rome Foundation. Rome III diagnostic criteria for functional
gastrointestinal disorders.
www.romecriteria.org/assets/pdf/19_RomeIII_apA_885-898.pdf. Accessed
December 23, 2011.
13. Shoemaker LK, Estfan B, Induru R, Walsh TD. Symptom management: an important part of cancer care. Cleve Clin J Med. 2011;78:25-34.
14. Hawley PH, Byeon JJ. A comparison of sennosides-based bowel
protocols with and without docusate in hospitalized patients with
cancer. J Palliat Med. 2008;11:575-581.
15. McNicol ED, Boyce D, Schumann R, Carr DB. Mu-opioid antagonists for opioid-induced bowel dysfunction. Cochrane Database Syst Rev. 2008;(2):CD006332.
16. Young-McCaughan S, Miaskowski C. Definition of and mechanism for opioid-induced sedation. Pain Manag Nurs. 2001;2:84-97.
17. Drugs for pain. Treat Guidel Med Lett. 2007;5:23-32.
18. Harris DG. Nausea and vomiting in advanced cancer. Br Med Bull. 2010;96:175-185.
19. Miller JL, Hagemann TM. Use of pure opioid antagonists for management of opioid-induced pruritus. Am J Health Syst Pharm. 2011;68:1419-1425.
20. Mishra S, Bhatnagar S, Nirvani Goyal G, et al. A comparative
efficacy of amitriptyline, gabapentin, and pregabalin in neuropathic
cancer pain: a prospective randomized double-blind placebo-controlled
study. Am J Hosp Palliat Care. 2011 Jul 10. [Epub ahead of print]
21. Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for
the pharmacological management of neuropathic pain: an overview and
literature update. Mayo Clin Proc. 2010;85:S3-S14.
22. McNicol E, Strassels SA, Goudas L, et al. NSAIDS or paracetamol, alone or combined with opioids, for cancer pain. Cochrane Database Syst Rev. 2005;(1):CD005180.
23. Stockler M, Vardy J, Pillai A, Warr D. Acetaminophen
(paracetamol) improves pain and well-being in people with advanced
cancer already receiving a strong opioid regimen: a randomized,
double-blind, placebo-controlled cross-over trial. J Clin Oncol. 2004;22:3389-3394.
24. US Food and Drug Administration. FDA drug safety communication:
prescription acetaminophen products to be limited to 325 mg per dosage
unit; boxed warning will highlight potential for severe liver failure.
www.fda.gov/Drugs/DrugSafety/ucm239821.htm. Accessed January 26, 2012.
To comment on this article, contact rdavidson@uspharmacist.com.





