US Pharm. 2020;45(9):27-29.
ABSTRACT: Since synthetic hormones were first combined in the 1940s, hormone replacement therapy has been widely used by women experiencing undesirable menopausal symptoms. Subsequent research led to discoveries of several negative effects associated with the use of hormone therapy, sparking discussions on the risks versus benefits of hormonal treatment. Because of the risks associated with commercial preparations, compounded hormone therapy (CHT) has become more prominent, as it has been touted as a biologically equivalent alternative to synthetic hormones. However, because of minimal to no FDA oversight in the compounding realm generally, CHT may not be as safe as has been advertised. In 2018, based on favorable trial results, Bijuva became the first FDA-approved bioidentical combination 17-beta estradiol-progesterone formulation.
For decades, women have turned to hormone replacement therapy to treat adverse vasomotor symptoms associated with menopause, such as hot flashes and night sweats. Women can spend up to one-third of their lives in a hormone-deficient state, which further contributes to such undesirable effects as mood changes, increased risk of osteoporosis, and decreased libido. Systemic estrogen is the most effective treatment for vasomotor symptoms, whereas genitourinary symptoms associated with menopause should be treated with low-dose topical estrogen.1 The observational Study of Women’s Health Across the Nation found that women experience vasomotor symptoms for an average of 7.4 years post menopause.2 However, most clinicians advise that treatment last for no longer than 5 years post menopause and strongly recommend avoiding hormone therapy altogether in women older than age 60 years.1 The clinician should carefully weigh the risks and benefits associated with synthetic and compounded hormones while keeping in mind the primary goal of improving the patient’s quality of life.
Bioidentical Hormones
Bioidentical hormones, such as estrogens, progestins, and androgens, are compounds that are prepared according to USP Chapter <795> Nonsterile Compounding standards. These hormones may be compounded in a variety of dosages and dosage forms, creating many options for women interested in compounded hormone therapy (CHT). Compounded formulations do not undergo the strict manufacturing and purity standards testing that their FDA-approved counterparts do; however, despite this unregulated environment, CHT is widely popular with patients seeking more “personalized” medicine.
The Endocrine Society has defined compounded bioidentical hormones as “compounds that have the exact same chemical and molecular structure as hormones that are produced in the human body.”3 The FDA asserts that the term bioidentical hormone is purely a marketing strategy, as there is no scientific basis for an implicit benefit or risk reduction for CHT over FDA-approved synthetic hormone therapy.3
Synthetic Hormone Therapy
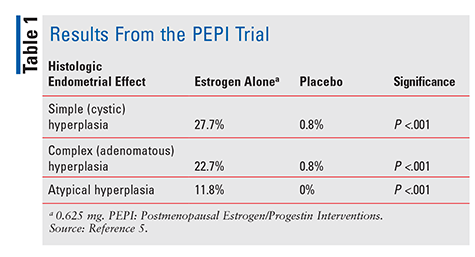
The FDA approved diethylstilbestrol in 1941 and conjugated equine estrogens (CEE) in 1942 to treat menopausal symptoms.4 Starting in the 1960s, estrogen sales skyrocketed until 1975, when reports surfaced that estrogen use could be associated with an increased risk of endometrial cancer.4 However, these reports were largely ignored, and estrogen use continued. Not until 1996, when the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial was published, did healthcare providers begin to truly understand the potential negative effects associated with estrogen therapy. PEPI was a 3-year randomized, placebo-controlled, double-blind, multicenter trial that examined the histologic endometrial effects of therapy with synthetic unopposed estrogen versus three different regimens of synthetic estrogen plus progestin.5
In the PEPI trial, significantly more subjects given estrogen alone developed simple, complex, or atypical endometrial hyperplasia versus subjects given placebo (TABLE 1). Furthermore, while the exact percentages were not specified, rates of hyperplasia in subjects administered one of the three estrogen-plus-progestin regimens were similar to those in subjects given placebo.5 In summary, administration of estrogen alone at a dosage of 0.625 mg per day led to a significantly increased incidence of endometrial hyperplasia, whereas combining CEE with progestin protected the endometrium from overgrowth to a considerable degree.5 Because of this proven endometrial-cancer risk, progestin is always given in combination with estrogen in women with an intact uterus. Further confirming this risk, a 2007 case series described three women who used CHT containing subtherapeutic doses of progestin and later developed endometrial cancer.6
The PEPI trial, along with results on 16,068 postmenopausal women from the Women’s Health Initiative (WHI) published in 2002, shed light on the serious concerns raised in the 1970s.7 Although the PEPI trial showed no increased risk of hyperplasia with the combination product, the WHI found significantly increased risks associated with synthetic hormone use, such as cardiovascular disease, stroke, thromboembolic events, and breast cancer.7 There was an 80% increased risk of cardiovascular disease at 1 year post discontinuation of combination CEE/medroxyprogesterone acetate therapy, and a 37% increased risk of ischemic stroke during the intervention phase with combination therapy. Also, breast-cancer risk was significantly increased (24%) during intervention. Estrogen-plus-progestin use did provide some benefits, with patients noting a decreased incidence of vaginal atrophy, reduced vasomotor symptoms such as hot flashes and night sweats, and a 38% decreased risk of colorectal cancer during treatment with combination therapy.7 Nonetheless, owing to the significance of the increased risk of breast cancer and thromboembolic events, the trial was stopped early.
Compounded Hormone Therapy
The risks discussed above paved the way for CHT advocacy because it was thought that tailoring or personalizing CHT to the individual patient would maximize the benefit and minimize the risk. Claims that CHT was safer and biologically friendlier became more prominent, and its use increased. Pinkerton and Santoro used two different surveys—Harris and Rose—to assess patients’ knowledge of CHT and quantify the use of CHT versus FDA-approved hormone therapy, respectively.8 Results of the Harris survey indicated that 86% of women aged 40 to 65 years were unaware that CHT was not FDA approved, and extrapolated results of the Rose survey suggested that, annually, up to 2.5 million women older than age 40 years may use CHT.8 Data from both surveys indicated that a physician or pharmacist had recommended CHT. These results suggest that an opportunity exists for providers to ensure that patients are informed about the product they are receiving, including all possible risks associated with hormone therapy, and for compounding pharmacists to ensure that they are delivering a therapeutically pure product that patients can trust.
Because the FDA is not responsible for standardization of compounded products, regulation often falls to state boards of pharmacy. These boards typically do not have the resources to conduct regular inspections of compounding pharmacies, and the FDA intervenes only when a complaint is made.9 Although compounded formulations are not legally required to carry risk warnings, theoretically these products may pose the same risks as those mentioned above for synthetic hormone therapy. To date, however, there have been no head-to-head safety trials of CHT versus synthetic hormones.10
There are three types of estrogens a woman may receive as part of a compounded hormone replacement regimen: estrone (E1), estradiol (E2), and estriol (E3), each having a varying degree of potency (E2 > E1 > E3).9 Because they vie for the same receptor, these estrogens act as competitive inhibitors, resulting in decreased efficacy if not correctly balanced.11 Consequently, proponents of CHT developed Biest (E2 + E3 in a 20/80 formulation) and Triest (E1 + E2 + E3 in a 10/10/80 formulation) in order to maximize physiologic potency, but there is currently no evidence to support the claim that these formulations in these ratios confer any safety benefit compared with synthetic hormone therapy.11
Although on the whole CHT has been inadequately studied and unregulated, in October 2018, based on findings from the REPLENISH trial, Bijuva became the first FDA-approved bioidentical combination 17-beta estradiol-progesterone formulation. REPLENISH, a phase III, randomized, double-blind, placebo-controlled, multicenter study, investigated the efficacy, endometrial safety, and overall safety of four different dosages of a single 17-beta estradiol-progesterone capsule for moderate-to-severe vasomotor symptoms in postmenopausal women.12 The primary safety endpoint was the incidence of endometrial hyperplasia at 12 months. At 12 months, no cases of endometrial hyperplasia were observed for any of the estradiol-progesterone dosage formulations.12 At 12 weeks, the estradiol 1 mg–progesterone 100 mg formulation demonstrated the largest statistically significant reduction in vasomotor symptom frequency and severity compared with placebo while maintaining primary safety-endpoint standards.12 However, as previously mentioned, there have been no trials comparing the safety of synthetic hormones versus bioidentical hormone formulations, so theoretically the risks remain.
Conclusion
Based on the REPLENISH trial, pre-, peri-, and postmenopausal women now have a standardized, regulated, bioidentical, FDA-approved treatment for vasomotor symptoms. Future trials are needed to fully delineate the safety profile of 17-beta estradiol and CHT. Pharmacists should recommend any oral hormone formulation for the shortest duration feasible. In addition, if CHT is chosen, pharmacists should ensure that their patients know what CHT is and how it differs from synthetic hormones while also having a frank conversation about the benefits and risks of hormone replacement therapy.
REFERENCES
1. Martin KA, Manson JE. Approach to the patient with menopausal symptoms. J Clin Endocrinol Metab. 2008;93(12):4567-4575.
2. Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531-539.
3. Santoro N, Braunstein GD, Butts CL, et al. Compounded bioidentical hormones in endocrinology practice: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2016;101(4):1318-1343.
4. Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med. 2005;118(suppl 12B):64-73.
5. Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275(5):370-375.
6. Eden JA, Hacker NF, Fortune M. Three cases of endometrial cancer associated with “bioidentical” hormone replacement therapy. Med J Aust. 2007;187(4):244-245.
7. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321-333.
8. Pinkerton JV, Santoro N. Compounded bioidentical hormone therapy: identifying use trends and knowledge gaps among US women. Menopause. 2015;22(9):926-936.
9. Bhavnani BR, Stanczyk FZ. Misconception and concerns about bioidentical hormones used for custom-compounded hormone therapy. J Clin Endocrinol Metab. 2012;97(3):756-759.
10. Bijuva (estradiol and progesterone) package insert. Boca Raton, FL: TherapeuticsMD, Inc; November 2019.
11. Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(suppl 1):3-63.
12. Lobo RA, Archer DF, Kagan R, et al. A 17beta-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132(1):161-170.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





