US Pharm.
2006;31(9):39-48.
Each
year, nearly 2% of U.S. women of repro ductive age have an induced
abortion.1 This sobering statistic reminds all health care
professionals that too often, our patients at risk for unintended pregnancy
either fail to use effective birth control or use effective methods
incorrectly. With the goal of helping pharmacists facilitate contraceptive
success for their patients, this review provides an update regarding hormonal
and intrauterine contraceptives and details newer methods, including the
progestin-releasing intrauterine system, the contraceptive patch and ring, and
extended-regimen oral contraceptives as well as emergency oral contraception.
Combined Hormone
Contraceptives
Developed more than
four decades ago and representing one of the best-studied prescription
medications, oral contraceptives (OCs) combining estrogen and progestin
continue to be the most popular reversible contraceptive used by women in the
U.S.2 OC use is safe for most women and is associated with numerous
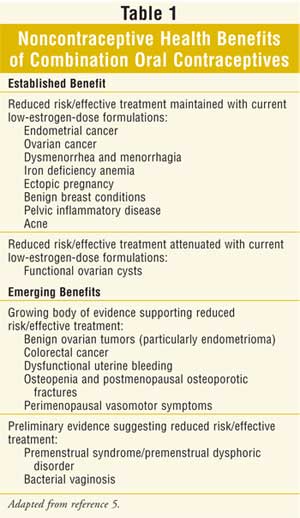
noncontraceptive benefits (Table 1). Although OCs represent a highly
effective birth control method when they are used correctly, inconsistent or
incorrect use (e.g., skipping as few as two to three consecutive days of use)
accounts for a surprisingly high annual failure rate of 8%.3,4 This
high failure rate underscores the clinical value of hormonal and intrauterine
contraceptives that do not require the users' daily attention.
Although the "pill" is not
new, many new approaches to initiating and taking OC tablets have recently
been developed. An innovative "quick start" approach to OC initiation involves
in-office ingestion of the first OC tablet immediately after a negative
pregnancy test, regardless of the patient's menstrual status. With this
method, patients have immediate exposure to combined hormonal contraceptives
(CHCs) and do not need to wait for their next menstrual period to begin or a
"Sunday start." A pilot study has suggested that this approach to OC
initiation is safe and results in higher rates of OC use than do conventional
pill initiation strategies. Patients who use the quick-start approach initiate
OC using an in-office sample pack but must fill their OC prescription in a
timely manner to ensure continued efficacy.5
The vast majority of
traditional OC regimens have used a 28-day plan with 21 days of combined
active pills followed by a seven-day hormone-free interval (HFI). A seven-day
HFI results in clearance of exogenous estrogen and progestin two to three days
after completion of active pills. This allows for several hormone-free days
during which levels of luteinizing hormone (LH) and follicle-stimulating
hormone (FSH) rise and follicular growth occurs with increased risk of
ovulation if active pills are not started on time (referred to as escape
ovulation).6 Shortening the HFI or adding low-dose estrogen
during the usual placebo interval may decrease the risk of escape ovulation
and further improve contraceptive efficacy.7 Previously, the only
OC (Mircette) that attempted to shorten the HFI substituted low-dose estrogen
(10 mcg ethinyl estradiol) for five of the seven usual placebo pills.8
In recent years, the trend
toward reduction or elimination of the traditional seven-day HFI or placebo
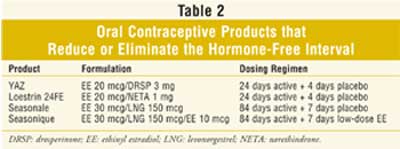
interval has resulted in the introduction of several new OC products (Table
2). Two new 28-day OC products, YAZ (3 mg drospirenone/20 mcg ethinyl
estradiol)and Loestrin 24 FE (1 mg norethindrone/20 mcg ethinyl estradiol),
were recently approved. Each provides 24, rather than 21, days of active
combined hormones, followed by four days of placebo tablets, thus shortening
the HFI.
The drosperinone-containing
product (YAZ) has also been evaluated in women with premenstrual dysphoric
disorder who desire pregnancy prevention. Response, defined as a 50%
improvement in daily mood and in physical and behavioral scores, was reported
in significantly more patients who received treatment in a placebo-controlled
study.9 Yet another OC product under development administers
combined OCs continuously without a pill-free interval for one year.
Extended OC regimens, which
reduce the number of withdrawal bleeding episodes per year, have also become
available and continue to increase in popularity. The first approved product
to use this approach was Seasonale (levonorgestrel 150 mcg/ethinyl estradiol
30 mcg), which extended active combined OC use to 84 days (12 weeks), followed
by one hormone-free week.10 Women interested in this "four periods
a year" approach to OCs need to be aware that breakthrough bleeding and
spotting (BTB/S) is more common during the initial active pill intervals than
that seen with conventional 28-day regimens. However, over time, unscheduled
BTB/S declines substantially.10
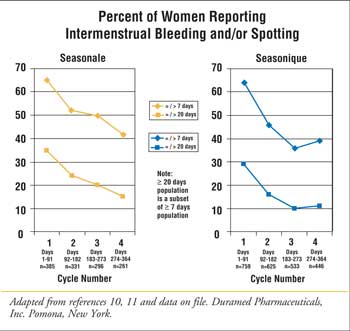
In May 2006, a 91-day-regimen
OC, Seasonique, was approved. This regimen is the first to completely
eliminate the HFI by utilizing seven days of low-dose estrogen (10 mcg ethinyl
estradiol) during the pill-free interval.11 While not evaluated in
head-to-head studies, bleeding data from clinical trials of the 91-day
regimens with and without low-dose estrogen during the HFI have been
subjectively compared (Figure 1).10,11 In each of the
studies, the incidence of BTB/S was similar in the initial cycle, as would be
expected given the use of equivalent formulations (levonorgestrel 150
mcg/ethinyl estradiol 30 mcg) during days 1 to 84. While the percentage of
patients reporting BTB/S decreased, the decrease was greater in the patients
who used low-dose estrogen during the HFI. The data for Seasonique showed a
median of less than one day of bleeding per patient month in extended cycles 2
through 4.11
It should be noted that women
taking extended-regimen CHCs, regardless of formulation or duration of
extension, will have a greater annualized exposure to estrogen compared to
women administered 28-day regimens. To date, there have been no reports of
additional health risks associated with this exposure.
Approved for contraceptive use
in 1992, depot medroxyprogesterone acetate (DMPA or Depo-Provera IM)
contraceptive injections provide reversible long-acting birth control that is
as effective as sterilization. In the 12 years since its introduction, DMPA
has been to a large degree responsible for declining rates of unintended
pregnancies and abortions among U.S. women, particularly teens.12
With more than three decades of experience with use abroad, an extensive and
reassuring safety track record has accumulated for DMPA.12 In
addition, use of DMPA is associated with noncontraceptive health benefits (
Table 3). However, in late 2004, the FDA added a black box warning to
DMPA's package labeling regarding loss of bone mineral density (BMD) in women
using the product. Because use of DMPA suppresses ovarian estradiol
production, BMD declines during DMPA use. Fortunately, BMD is regained rapidly
after discontinuation of DMPA, and studies of BMD in women and adolescents who
had discontinued use of the product showed no BMD deficit compared to women
who have never used DMPA.5 DMPA use has not been linked to the
occurrence of osteoporosis or fractures. Accordingly (and notwithstanding the
black box), concerns regarding BMD should not limit prescription or
continuation of DMPA. Furthermore, ordering dual x-ray absorptiometry testing
in healthy young women receiving DMPA is not appropriate.13 When
DMPA is being used or considered in women with specific risk factors for low
BMD (e.g., slender athletes, perimenopausal smokers), use of supplemental
estrogen (e.g., oral conjugates equine estrogen 0.625 mg daily or equivalent
doses of other oral/transdermal estrogen) is appropriate.13

A new low-dose subcutaneous
formulation of DMPA, depo-subQprovera104 is now available.14
Medroxyprogesterone acetate injectable suspension (DMPA-SC) administered once
every three months provides a 30% lower total dose than traditional DMPA IM
(150-mg injection). In clinical studies, it suppressed ovulation for more than
13 weeks in all subjects regardless of body mass index, with no pregnancies
reported in more than 16,000 woman cycles of use.5,14 Although not
evaluated in a head-to-head study, the incidence of bleeding and amenorrhea
reported with DMPA-SC appeared to be similar to that reported with DMPA.
Since 2000, two alternative
estrogen-progestin CHC methods and one intrauterine contraceptive have been
introduced in the U.S. (Table 4). Approved in 2002, the contraceptive
patch, Ortho-Evra (transdermal norelgestromin/ethinyl estradiol patch), is
applied once weekly for three weeks; subsequently, withdrawal bleeding is
anticipated during a patch-free week. In a randomized clinical trial, the
contraceptive efficacy of the patch was comparable to that of oral
contraception.5 Application site reactions, breast discomfort, and
dysmenorrhea were each significantly more common in women treated with the
patch. This may, in part, be explained by a significantly higher overall
estrogen exposure.15 In a randomized, open-label pharmacokinetic
study, mean area under the time versus concentration curve 0–24 hours for the
patch was 1.6 times higher than reported for combination OC therapy containing
30 mcg ethinyl estradiol (p <0.05). Although this observation led to the FDA
adding a warning to the prescribing information for the contraceptive patch,
available published epidemiologic data indicates that the risk of venous
thromboembolism in users of the patch is similar to that in women using
combination OCs.16,17
Contraceptive efficacy with
the patch may be compromised in heavier women (>90 kg/198 lbs). In clinical
trials, women weighing at least 198 pounds had a statistically significantly
higher rate of pregnancy than the population of lower-weight women.16

Also introduced in the U.S. in
2002, NuvaRing (etonogestrel/ethinyl estradiol vaginal ring) is worn for three
weeks, followed by one ring-free week during which withdrawal bleeding occurs.
In comparative clinical trials, rates of breakthrough bleeding and spotting
were lower with the ring than with OCs.5 More than three quarters
of users indicated that they did not note discomfort related to the ring in
general or during sexual relations.
Studies have also been
conducted on extended use of Ortho Evra and Nuva Ring.18,19 In the
study of the patch, patients randomized to the 91-day cycle reported fewer
bleeding days but more spotting days during active therapy and significantly
longer menstrual cycles (6.9 days compared to 5.2 days; p <.001) than did
women in the 28-day–cycle group.18 In the vaginal ring
extended-regimen study, women randomized to the shorter extended regimen (49
days) experienced less bleeding and/or spotting than did women who received
the 91-day or 364-day regimens.19
Intrauterine Contraception
Widely used worldwide, intrauterine
devices (IUDs) offer women convenient, reversible birth control as effective
as surgical sterilization. In the mid-1970s, IUDs accounted for nearly 10% of
birth control methods use by U.S. women;5 however, today only 1% of
U.S. women use IUDs. This decline can be largely attributed to concerns among
clinicians and women regarding the link between IUDs and salpingitis and tubal
infertility; in particular, the morbidity and litigation associated with the
flawed Dalkon Shield has alarmed many people. A high-quality systematic review
found that if any increase in the risk of salpingitis is associated with IUD
use, it is small and appears confined to the first 20 days to one month
post-insertion and falls thereafter.20 Risk is also higher among
women with multiple sexual partners. Likewise, use of an IUD is not associated
with a subsequent increased risk of tubal infertility.20,21
Knowledge of these risks and their management may encourage clinicians to
recommend use of IUDs more broadly.
Two IUDs are currently
marketed in the US: Mirena (Table 5) and ParaGard. The copper IUD
(ParaGard), introduced in 1988 and approved for up to 10 years of use, is a
good choice for women who cannot or prefer not to use hormonal contraception.
Because use of the copper IUD can increase menstrual flow and cramps, it is
appropriate for women who have no excess menstrual flow or cramps at baseline.
In the fall of 2005, the FDA revised prescribing information for the copper
IUD.22 Nulliparous women (i.e., those who have never been
pregnant), as well as women with a history of pelvic inflammatory disease are
now considered appropriate candidates; however, women at current high risk for
sexually transmitted infection remain poor candidates. The IUD may be inserted
immediately post-partum or after the second month post-partum and is safe for
use during lactation.

The levonorgestrel-releasing
intrauterine system (IUS; Mirena), introduced in 2000 and approved for up to
five years of use, reduces menstrual flow and is therefore appropriate for
women who can use a progestin-based method and desire long-term contraception.
In addition to use as a contraceptive method, it has also been used in the
management of heavy menstrual bleeding and may be a more cost-effective
alternative to hysterectomy.23 However, women interested in using
the levonorgestrel-releasing IUS should be aware of that initial irregular
spotting or bleeding is common after insertion of this device. Hormonal side
effects, including acne and ovarian cysts, also occur in some users.
Noncontraceptive benefits associated with use of the levonorgestrel-releasing
IUS are detailed in Table 5.
Emergency Contraception
The use of
emergency (post-coital) contraception (EC) is estimated to have averted more
than 51,000 induced abortions in 2000.1 The only dedicated EC
formulation available in the U.S. is the progestin-only formulation (Plan B),
which consists of two levonorgestrel 0.75-mg tablets.
Progestin-only EC is more
effective and causes less nausea and emesis than combination EC.24
Prescribing information for Plan B indicates one tablet should be taken within
72 hours of unprotected intercourse, followed by a second tablet 12 hours
later.25 However, taking the two tablets together may be as
effective in preventing pregnancy as dividing the dose.24
Optimally, Plan B should be taken as soon as possible or within 72 hours of
unprotected intercourse; however, there is evidence that Plan B retains its
efficacy in pregnancy prevention when it is taken up to five days after
unprotected intercourse.24
In August, the FDA announced
that Plan B has been approved for OTC use in women ages 18 and older. The
product will remain available by prescription only for women ages 17 and
younger. Duramed, Plan B's manufacturer and a Barr subsidiary, has agreed to
provide consumers and health care professionals with labeling and education on
the product, as well as a hotline that provides answers to questions on Plan
B. Plan B will be available OTC only in locations staffed by licensed health
care professionals and stocked behind the counter, because it cannot be
dispensed without proof of age.
Pharmacists have a critical
role in providing access to EC, most importantly by maintaining an inventory
of Plan B. Pharmacists can provide women with information about the efficacy
and possible side effects of EC, as well as the need to obtain follow-up care
if menses do not occur as expected.
Use of Hormonal
Contraception and Risk of Breast Cancer
Since the July 2002
publication of findings from the Women's Health Initiative (WHI), which found
an increased breast cancer risk with use of menopausal combination hormone
therapy, concerns that use of combination hormonal contraception might also
increase this risk have been heightened. Many health care professionals are
not aware of the Women's CARE study, a large study sponsored by the CDC and
NIH that examined breast cancer risk associated with use of hormonal
contraceptives.26 Initial findings, published in August 2002,
indicated that regardless of length of OC therapy and age or family history,
use of OCs was not associated with an elevated breast cancer risk.5,26
A more recent publication from this same study found that neither use of DMPA
nor progestin-only implants is linked with an increase in breast cancer risk.
27
Conclusion
Given the number
and range of new contraceptive options, it is clear that birth control has
expanded well beyond the pill.5 Pharmacists knowledgeable about
older hormonal and intrauterine contraceptives and new longer-acting and
emergency contraceptives can have an important role by counseling their
patients and positively impacting public health by minimizing unintended
pregnancy and induced abortion.
Acknowledgement: The author acknowledges
the invaluable assistance of Kathryn M. Martin, PharmD, Ms. Georgette
Andreason, and Ms. Pam Neumann.
References
1. Jones RK, Darroch JE, Henshaw SK. Contraceptive use among U.S. women having
abortions in 2000-2001. Perspect Sex Reprod Health. 2002;34:294-303.
2. National Center for Health Statistics. Primary contraceptive methods among
women aged 15–44 years--United States, 2002. JAMA.
2005;293:2208.
3. Petitti DB. Combination
estrogen-progestin oral contraceptives. N Engl J Med.
2003;349:1443-1450.
4. Trussel J. Contraceptive failure in the United States. Contraception
. 2004;70:89-96.
5. Kaunitz AM. Beyond the pill: new data and options in hormonal and
intrauterine contraception. Am J Obstet Gynecol. 2005;192:998-1004.
6. Baerwald AR, Olatunbosun OA, Peirson RA. Ovarian follicular development is
initiated during the hormone-free interval of oral contraceptive use.
Contraception. 2004;70:371-377.
7. Mishell DR. Rationale for decreasing the number of days of the hormone-free
interval with use of low-dose oral contraceptive formulations. Contraception
. 2005;71:304-305.
8. Mircette Study Group. An open-label, multicenter, noncomparative safety and
efficacy study of Mircette, a low-dose estrogen-progestin-oral contraceptive.
Am J Obstet Gynecol. 1998;179:S2-S8.
9. Yonkers KA, Brown C, Pearlstein RB, et al. Efficacy of a new low-dose oral
contraceptive with
drosperinone in premenstrual dysphoric disorder. Obstet Gynecol.
2005;105:492-501.
10. Anderson FD, Hait H. A multicenter, randomized study of an extended cycle
oral contraceptive. Contraception. 2003;68:89-96.
11. Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended
regimen oral contraceptive using continuous low-dose ethinyl estradiol.
Contraception. 2006;73:229-234.
12. Westhoff C. Depot-medroxyprogesterone acetate injection (Depo-Provera®); a
highly effective contraceptive option with proven long-term safety.
Contraception. 2003;68:75-87.
13. Kaunitz AM. Depo-Provera's black box: Time to reconsider? Editorial.
Contraception. 2005;72:165-167.
14. Jain J, Jakimiuk AJ, Bode FR, et al. Contraceptive efficacy and safety of
DMPA-SC. Contraception. 2004;70:269-275.
15. van den Heuvel MW, van Bragt AJM, Alnabawy AKM, Kaptein MCJ. Comparison of
ethinylestradiol pharmacokinetics in three hormonal contraceptive
formulations: the vaginal ring, the transdermal patch and an oral
contraceptive. Contraception. 2004;72:168-174.
16. Ortho Evra [prescribing information]. Ortho-McNeil Pharmaceuticals,
Raritan, NJ. Available at:
www.orthoevra.com/active/janus/en_US/assets/common/company/pi/orthoevra.pdf#zoom=100.
Accessed June 7, 2006.
17. Jick SS, Kaye JA, Russman S, Jick H. Risk of nonfatal venous
thromboembolism
in women using a
contraceptive transdermal patch and oral contraceptives containing
norgestimate and 35 microg of ethinyl estradiol. Contraception.
2006;73:223-238. Epub 2006 Jan 26.
18. Stewart FH, Kaunitz AM, Laguardia KD, et al. Extender use of transdermal
norelgestromin/ethinyl estradiol: a randomized trial. Obstet Gynecol.
2005;105:1389-1396.
19. Miller L, Verhoeven CH, Hout JL. Extended regimens of the contraceptive
vaginal ring: a randomized trial. Obstet Gynecol. 2005;106:473-482.
20. Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet
. 2000;356:1013-1019.
21. Hubacher D, Lara-Ricalde R, Taylor DJ, et al. Use of copper intrauterine
devices and the risk of infertility among nulligravid women. New Eng J Med
. 2001;345:561-567.
22. ParaGard T380A Intrauterine Copper Contraceptive [prescribing
information]. Duramed Pharmaceuticals, Woodcliff Lake, NJ. Available at:
www.paragard.com/paragard/custom_images/Prescribing-Info.pdf. Accessed June 8,
2006.
23. Lethaby AE, Cooke I, Rees M. Progesterone or progestogen-releasing
intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst
Rev. 2005; 19(4):CD002126.
24.Westhoff C. Emergency Contraception. N Engl J Med 2003;
349:1830-1835.
25. Plan B [prescribing information]. Duramed Pharmaceuticals, Pomona, NY.
Available at: www.go2planb.com/PDF/PlanBPI.pdf. Accessed June 8, 2006.
26. Marchbanks PA, McDonald JA, et al. Oral contraceptives and the risk of
breast cancer. N Engl J Med. 2002;346: 2025-2032.
27. Strom BL, Berlin JA, Weber AL, et al. Absence of an effect of injectable
and implantable progestin-only contraceptives on subsequent risk of breast
cancer. Contraception. 2004;69:353-360.
To comment on this article, contact
editor@uspharmacist.com.






