US Pharm. 2023;48(9):17-25.
ABSTRACT: There are a number of contraceptive options, both hormonal and nonhormonal, available in a variety of formulations (e.g., implant, pill, patch) to prevent pregnancy. This article will review the categories of contraceptives, including advantages and disadvantages, in decreasing order of efficacy. Hormonal contraceptives have notable drug interactions that can reduce contraceptive efficacy. Given the prevalence of contraceptives, the variety of products, and varying degrees of efficacy, it is critical for pharmacists to understand the nuances associated with each contraceptive to best educate their patients.
Prior to initiating any hormonal contraceptive, patients should be evaluated for pregnancy and have an in-depth discussion surrounding the benefits and risks. Contraindications to estrogen-containing contraceptives include women aged >35 years who smoke tobacco; history of venous thromboembolism (VTE), stroke, coronary artery disease (CAD), hypercoagulable state, or thrombosis of heart valves; history of liver, breast, or ovarian cancer; liver disease; migraine with aura; uncontrolled hypertension; and diabetes with vascular disease.1 Due to the increased risk of thrombosis associated with estrogen, patients should be counseled on abdominal pain, chest pain, headaches, eye problems, and severe leg pain (ACHES).2 Contraindications to progestin-containing contraceptives include breast, liver, or progestin-sensitive cancer and liver disease. Because hormonal contraceptives do not protect against sexually transmitted infections (STIs), condom use is encouraged (with the exception of natural-membrane condoms, which are labeled to prevent pregnancy only).1
Implant
The implant is a long-acting reversible contraceptive (LARC) that is a highly effective and safe contraceptive option for patients. Nexplanon is a matchstick-sized rod containing 68 mg of etonogestrel (ENG) inserted into the underside of the nondominant arm by a healthcare provider.4
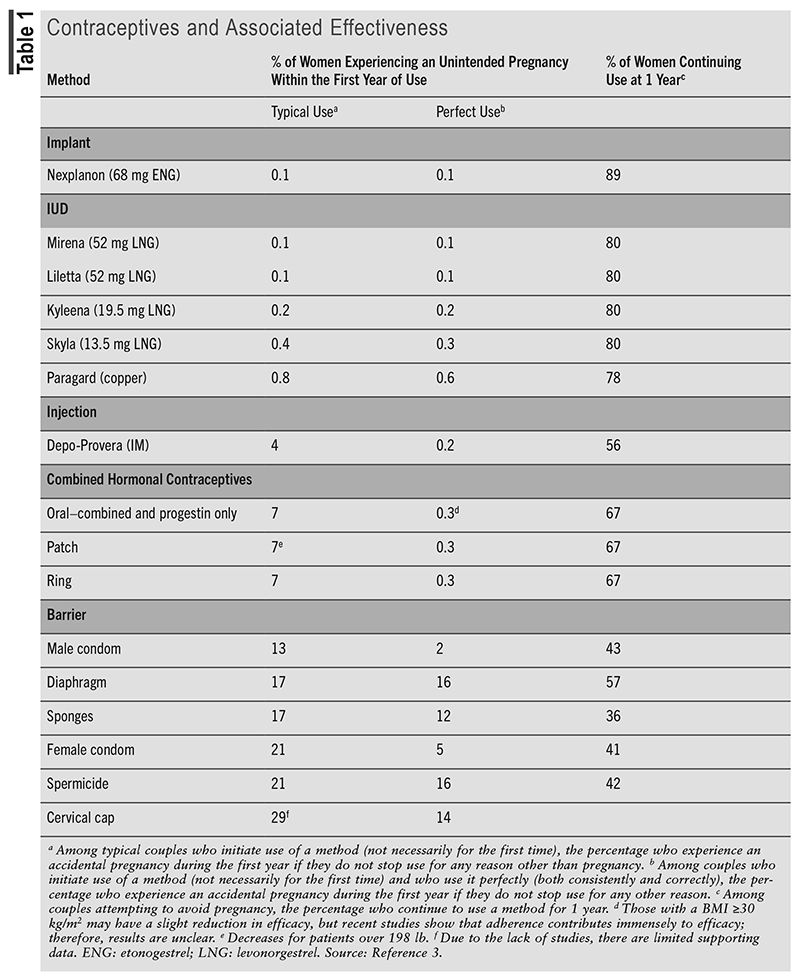
Nexplanon is the only contraceptive implant available in the United States and is currently approved for up to 3 years.4 Advantages of the ENG implant include its reversibility, effectiveness (see TABLE 1), and long-term use. Once removed, there is a rapid return of fertility, typically within two menstrual cycles.5 Efficacy is not compromised by BMI, unlike with some forms of contraception. The most prominent noncontraceptive benefit of the ENG implant is a significant reduction in dysmenorrhea. One study reported that 77% of patients with baseline dysmenorrhea described complete resolution of their symptoms with the ENG implant.6
Minor procedure-related risks include localized bleeding, redness, swelling, bruising, and discomfort. If the implant is inserted >5 days after the onset of menses, a backup nonhormonal contraceptive should be used for 7 days. Patients often experience unpredictable bleeding, ranging from spotting or light bleeding to amenorrhea, which may lead to discontinuation.3 In contrast to intrauterine devices (IUDs), the ENG implant relies on systemic absorption, increasing the occurrence of adverse effects, including lack of libido, headaches, weight gain, acne, breast pain, changes in mood, vaginitis, and gastrointestinal issues.4,6-9
Intrauterine Devices
In the U.S., IUDs are the most commonly used LARC, with studies reporting continued increase in use due to their high efficacy and safety.10 Notable differences among IUDs are the active ingredient, copper (Cu) versus levonorgestrel (LNG), and their effect on menstrual bleeding. IUDs are inserted directly through the cervix into the uterus by a healthcare provider with the option for self-removal.
Five IUDs are currently available, each made from a T-shaped polyethylene frame with barium sulfate, allowing for x-ray imaging. Paragard, the Cu-IUD, is the only hormone-free option and is FDA approved for 10 years. The hormonal IUDs each contain LNG. Mirena and Liletta have the same-size frame containing 52 mg of LNG with approval for 5 and 3 years, respectively. Kyleena and Skyla have smaller frames that contain 19.5 and 13.5 mg of LNG with approval for 5 and 3 years, respectively.11
The primary advantage of IUDs is their high efficacy (see TABLE 1). Similar to the implant, there is a rapid return of fertility after IUD removal, typically within two menstrual cycles.5 A potential advantage of the higher-dose LNG-IUDs is the occurrence of oligomenorrhea or amenorrhea in 50% of patients after 2 years.12 In comparison, the lower-dose LNG-IUDs frequently shorten and regulate menses. Due to minimal systemic absorption, IUDs are associated with fewer adverse effects (e.g., nausea, breast tenderness, fatigue, weight gain). The Cu-IUD does not require a backup nonhormonal contraceptive after placement, unlike its hormonal counterpart.11
Insertion is commonly associated with pain, discomfort, dizziness, and nausea. Following placement, patients may experience cramping for the next few days that can be managed with OTC pain medications. If an LNG-IUD is placed >7 days after the start of menstrual bleeding, a backup nonhormonal contraceptive should be used for 7 days. Irregular spotting or light bleeding is common for several months following LNG-IUD placement, whereas heavy or prolonged bleeding may occur following Cu-IUD insertion. Heavy bleeding and dysmenorrhea are common causes for discontinuation of the Cu-IUD, while amenorrhea and spotting are more likely causes for LNG-IUD discontinuation. Although perforation or expulsion is rare, patients should routinely check for the presence of the IUD strings.11 IUDs are associated with an increased risk of pelvic inflammatory disease (PID) for 20 days following insertion; the risk then returns to baseline, therefore not increasing the prevalence of PID long-term.13 Patients with an active pelvic infection, such as PID or endometriosis, or who are awaiting treatment for cervical and/or endometrial cancer should not have an IUD inserted.
Injection
Depo-Provera (depot medroxyprogesterone acetate [DMPA]) is an injectable hormonal contraceptive administered either intramuscularly (IM) or subcutaneously (SC) depending on the product. It is administered every 3 months by a healthcare provider (IM) or the patient (SC).14
Two DMPA products are currently available, distinguishable by their route of administration: SC (Depo-SubQ Provera 104; DMPA-SQ) and IM (Depo-Provera; DMPA-IM). Depo-SubQ Provera 104 contains 104 mg of DMPA administered SC in the upper anterior thigh or abdomen every 12 to 14 weeks. Depo-Provera contains 150 mg of DPMA administered IM in the gluteal or deltoid muscle every 13 weeks.14
The dosing interval ranges from 12 to 14 weeks with a 2-week grace period for late injections. Repeat injections may be given early to avoid a missed dose. Continued use (>1 year) of DMPA may result in amenorrhea or bleeding irregularities. The DMPA-SQ product can be self-administered at home, providing convenience and privacy to patients.14
The DMPA injection is associated with significant gynecological bleeding, which is highest during the first year, including irregular or unpredictable bleeding, spotting, and heavy or prolonged bleeding. The bleeding irregularities are more common with the IM product and frequently result in discontinuation.15 The DMPA injection is associated with significant weight gain, upwards of 10 kg after 2 to 4 years of continued use, a common reason for discontinuation.16 A transient reduction in bone mineral density (BMD) has been reported, with the most significant impact occurring after 2 years of use; therefore, calcium and vitamin D supplementation are recommended. Unfortunately, BMD recovery can take several years after discontinuation of DMPA.17,18 Compared with all other contraceptives, the injection is associated with the longest delay in return of normal fertility, approximately five to eight menstrual cycles.5 If DMPA is started >7 days after the start of menstrual bleeding, a backup nonhormonal contraceptive should be used for 7 days.11
Oral (Combined and Progestin-Only)
Combined oral contraceptives (COCs) contain estrogen and progestin in a small tablet that is dosed once daily for 21 to 24 days, followed by 4 to 7 days of placebo tablets to allow for menses.19 Progestin-only pills (POPs) or minipills are taken orally once daily; unlike COCs, they do not have a placebo week.20 Compared with the implant, IUDs, and injection, COCs and minipills have reduced efficacy, likely owing to their dependency on patient adherence (TABLE 1).19,20
COC formulations are classified as mono-, bi-, tri- or quadriphasic, depending on the number of dose changes in each pill pack (e.g., monophasic formulations have a consistent dose of estrogen and progestin). Currently available minipill packs contain 28 days of 0.35-mg norethindrone (NET).19,20
COCs may improve acne, dysmenorrhea, irregular menses, and heavy bleeding; extended-cycle dosing avoids monthly menses. Minipills are associated with fewer adverse effects and possess fewer contraindications than COCs due to their progestin-only composition. Minipills are advantageous for breastfeeding patients, as estrogen can impact milk supply, and for those who cannot safely take estrogen (e.g., migraine with aura).19,20 Both COCs and minipills have a relatively quick return of normal fertility, roughly three menstrual cycles.5
The effectiveness of oral hormonal contraceptives relies on correct use and patient adherence. Due to the low dose of progestin, the minipill must be taken at the same time every day within a 3-hour window. For patients prescribed minipills, it is important to take a missed dose as soon as it is remembered. If >3 hours elapse since the scheduled dose or the patient experiences vomiting or diarrhea within 4 hours of administration, a backup nonhormonal contraceptive should be used for 48 hours. Similar to POPs, COC missed doses should be taken as soon as remembered. If a patient misses ≥2 COC doses, then a backup nonhormonal contraceptive should be used for 7 days. If a COC or minipill is started >5 days after the start of menstrual bleeding, a backup nonhormonal contraceptive should be used for 7 and 2 days, respectively. The minipill should be started no sooner than 5 days following ulipristal, an emergency contraceptive (EC), due to an increased chance of pregnancy. Common side effects are nausea, irregular bleeding, headache, weight changes, changes in mood, and fatigue.19,20
Patch
Patch contraceptives are combination hormonal contraceptives (CHCs) containing both estrogen and progestin. Each patch should be replaced weekly for 3 weeks followed by a patch-free week to allow for menses. Patches should be applied to clean, dry, intact skin on the back, abdomen, buttocks, or upper arm.19
Currently available contraceptive patches in the U.S. include ethinyl estradiol (EE) 35 mcg/norelgestromin, 150 mcg (Xulane, Zafemy), and EE 30 mcg/LNG 120 mcg (Twirla).19 The contraceptive patch can improve acne, dysmenorrhea, irregular menses, and heavy bleeding. Contraceptive patches can be easily applied by the patient and offer convenience, considering they are applied once per week.19 The patch may be removed at any time and takes approximately four menstrual cycles for normal fertility to return.5
The effectiveness of the patch is dependent on correct use and patient adherence, as the patient must remember to change the patch on the same day each week. Common side effects are skin irritation, nausea, irregular bleeding, headache, weight fluctuations, changes in mood, and fatigue. Their use is contraindicated in patients with a BMI ≥30 kg/m2 due to increased risk of contraceptive failure.21 If the patch is applied >24 hours after the start of menstrual bleeding, a backup nonhormonal contraceptive should be used for 7 days. If detachment occurs or delayed application exceeds 2 days, a backup nonhormonal contraceptive should be used for 7 days.19
Vaginal Ring Contraceptives
Vaginal ring contraceptives are CHCs consisting of a latex-free, flexible ring that is inserted vaginally. The ring is inserted by the patient and remains in place for 3 weeks, followed by a 1-week ring-free period to allow for menses, after which a new ring is placed.19
Three contraceptive rings are currently available in the U.S.—ethinyl estradiol (EE) 0.015 mg/0.12 mg; etonogestrel (EluRyng, NuvaRing, and Haloette); and EE 0.013 mg/segesterone acetate 0.15 mg (Annovera).19
The contraceptive ring can improve acne, dysmenorrhea, irregular menses, and heavy bleeding. NuvaRing and EluRyng may be left in place for 4 weeks (extended-cycle) to avoid monthly menses. The vaginal ring offers convenient dosing and can be inserted and removed by the patient in the privacy of her home.19 The ring may be removed at any time and takes approximately three cycles for normal fertility to return.5
Common side effects include nausea, irregular bleeding or spotting, headache, and breast tenderness. If Annovera is inserted >5 days or EluRyng, NuvaRing, and Haloette are inserted >24 hours after the start of menstrual bleeding, a backup nonhormonal contraceptive should be used for 7 days. It is important for patients to replace the ring as soon as it is remembered. If the vaginal ring is displaced or insertion is delayed >2 hours for Annovera or >3 hours for EluRyng, NuvaRing, and Haloette, a backup nonhormonal contraceptive should be used for 7 days.22,23
Barrier Methods
While barrier contraceptives are commonly used, they are the least effective method owing to the need for correct and consistent use. Spermicide can be used alone or with another barrier contraceptive to improve efficacy (except the sponge, as it already contains spermicide).24
Barrier contraceptives include male and female condoms, cervical caps, diaphragms, and select nonoxynol-9-containing spermicides (sponges, gels, and foams). Diaphragms and cervical caps require a prescription, while all other products are available OTC.24
Barrier contraceptives are cost effective, and the majority are readily available without a prescription (e.g., condoms, spermicides, and sponges). Condoms are the only contraceptive method that may protect against STIs, including HIV. Male latex condoms offer the best protection, though nonlatex male condoms made of polyurethane or polyisoprene may be recommended for patients who are allergic to latex. Notably, natural membrane condoms made of lamb cecum do not offer STI protection. Barrier contraceptives offer excellent options for individuals who are unable to tolerate or use other contraceptives.24
Patients must be cognizant of which lubricants are compatible with condoms to decrease risk of weakening or breakage. Additionally, sponges, cervical caps, and diaphragms should not be used during menses or by patients with history of toxic shock syndrome (TSS). Depending on the product, potential side effects include TSS, vaginal burning and irritation, and urinary tract infections. Patients who have recently given birth are required to wait at least 6 weeks prior to inserting a new sponge, cervical cap, or diaphragm. Patients allergic to latex, polyurethane, or silicone could have a potential reaction to condoms or cervical caps.24
Emergency Contraceptives
ECs are used after unprotected intercourse to prevent unintended pregnancy. Common indications include contraceptive failure or lack of use. ECs should not be used as routine contraception, do not protect against STIs, and cannot terminate an existing pregnancy.25,26
Four EC options are available: the Cu-IUD and three emergency contraception pills (ECPs). The common-practice ECPs are ulipristal (UPA) 30 mg taken as a single dose and LNG 1.5 mg taken as a single dose or 0.75 mg taken twice 12 hours apart. Less commonly, Yuzpe (EE 100 mcg/LNG 0.5 mg) taken twice 12 hours apart can also be utilized. LNG is available OTC.25,26
The Cu-IUD is highly effective (99.9%) when used within 120 hours (5 days) of ovulation following unprotected intercourse. Efficacy of ECPs decreases as time from intercourse increases. When taken correctly, UPA possesses the highest efficacy followed by LNG. Yuzpe, being the least effective ECP, has fallen out of favor.25,26
Compared with other ECs, LNG is accessible to patients given its availability OTC. UPA and the Cu-IUD methods are not impacted by patient weight. Use of an ECP does not negatively affect a patient’s ability to conceive at a later time.25,26
LNG is affected by body weight and may be less effective for patients with a BMI ≥30 kg/m2. Common side effects include nausea and vomiting, headache, abdominal pain, fatigue, dizziness, and breast tenderness.25,26
Conclusion
The wide variety of hormonal and nonhormonal contraceptives allow for unique product selection specific to patient preference. LARCs have the highest efficacy but require an insertion procedure that may be uncomfortable. While the injection is dosed every 3 months, it is associated with significant weight gain, reduced BMD, and delayed return of fertility. Oral options, both COCs and minipills, are easy to administer but have lower rates of adherence. The vaginal ring and patch offer convenient dosing; however, the patch can cause skin irritation. Lastly, barrier contraceptives must be properly placed prior to anticipated sexual intercourse, which may be inconvenient. To properly educate patients, it is imperative that pharmacists have a thorough understanding of the available contraceptives and their associated nuances, including drug interations (TABLE 2).27,28

REFERENCES
1. Cooper DB, Patel P, Mahdy H. Oral contraceptive pills. In: StatPearls. Treasure Island, FL: StatPearls Publishing; November 24, 2022.
2. Seibert C, Barbouche E, Fagan J, et al. Prescribing oral contraceptives for women older than 35 years of age. Ann Intern Med. 2003;138:54-64.
3. Hatcher RA. Contraceptive Technology. Ayer Company Publishers, Inc.; 2018.
4. Nexplanon package insert. Bethesda, MD: U.S. National Library of Medicine; 2022 Nov [updated 2022 Nov]. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=487f8a62-e142-457c-97cc-2e398fde7594. Accessed June 15, 2023.
5. Yland JJ, Bresnick KA, Hatch EE, et al. Pregravid contraceptive use and fecundability: prospective cohort study. BMJ. 2020;371:m3966.
6. Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203(2):1-7.
7. Blumenthal PD, Gemzell-Danielsson K, Marintcheva-Petrova M. Tolerability and clinical safety of Implanon. Eur J Contracept Reprod Health Care. 2008;13(suppl 1):29-36.
8. Mansour D, Korver T, Marintcheva-Petrova M, et al. The effects of Implanon on menstrual bleeding patterns. Eur J Contracept Reprod Health Care. 2008;13(suppl 1):13-28.
9. CDC. Implants. March 27, 2023. www.cdc.gov/reproductivehealth/contraception/mmwr/spr/implants.html#followup. Accessed June 29, 2023.
10. Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among U.S. women, 2009–2012. Obstet Gynecol. 2015;126:917-927.
11. CDC. Intrauterine Contraception. March 27, 2023. www.cdc.gov/reproductivehealth/contraception/mmwr/spr/intrauterine.html. Accessed June 29, 2023.
12. American College of Obstetricians and Gynecologists. Committee on Clinical Consensus–Gynecology. General approaches to medical management of menstrual suppression. Obstet Gynecol. 2022;140(3):528-541. www.acog.org/-/media/project/acog/acogorg/clinical/files/clinical-consensus/articles/2022/09/general-approaches-to-medical-management-of-menstrual-suppression.pdf.
13. Farley TM, Rosenberg MJ, Rowe PJ, et al. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785-788.
14. CDC. Injectables. March 27, 2023. www.cdc.gov/reproductivehealth/contraception/mmwr/spr/injectables.html. Accessed June 29, 2023.
15. Lexicomp online. Medroxyprogesterone acetate. 2023. http://online.lexi.com.fi.opal-libraries.org/lco/action/home. Accessed June 29, 2023.
16. Lopez LM, Ramesh S, Chen M, et al. Progestin-only contraceptives: effects on weight. Cochrane Database Syst Rev. 2016(8):CD008815.
17. Curtis KM, Martins SL. Progestogen-only contraception and bone mineral density: a systematic review. Contraception. 2006;73(5):470-487.
18. American College of Obstetricians and Gynecologists. Committee on Adolescent Health Care. Committee on Gynecologic Practice. ACOG Committee Opinion No. 602: Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2014;123(6):1398-1402.
19. CDC. Combined hormonal contraceptives. March 27, 2023. www.cdc.gov/reproductivehealth/contraception/mmwr/spr/combined.html. Accessed June 29, 2023.
20. CDC. Progestin-only pills. March 27, 2023. www.cdc.gov/reproductivehealth/contraception/mmwr/spr/progestin.html. Accessed June 29, 2023.
21. Kardos L. Levonorgestrel emergency contraception and bodyweight: are current recommendations consistent with historic data? J Drug Assess. 2020;9(1):37-42.
22. Lexicomp online. Ethinyl estradiol and etonogestrel. 2023. http://online.lexi.com.fi.opal-libraries.org/lco/action/home. Accessed June 29, 2023.
23. Lexicomp online. Segesterone acetate and ethinyl estradiol. 2023. http://online.lexi.com.fi.opal-libraries.org/lco/action/home. Accessed June 29, 2023.
24. Integris Health. Know your birth control options: part 1: barrier methods. Female Health. 2018; https://integrisok.com/resources/on-your-health/2018/september/know-your-birth-control-options-barrier-methods.
25. CDC. Emergency contraception. March 27, 2023. www.cdc.gov/reproductivehealth/contraception/mmwr/spr/emergency.html. Accessed June 29, 2023.
26. American College of Obstetricians and Gynecologists. Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 152: Emergency Contraception. Obstet Gynecol. 2015;126(3):e1-e11.
27. Le Corvaisier C, Capelle A, France M, et al. Drug interactions between emergency contraceptive drugs and cytochrome inducers: literature review and quantitative prediction. Fundam Clin Pharmacol. 2021;35(2):208-216.
28. Aronson JK, Ferner RE. Analysis of reports of unintended pregnancies associated with the combined use of non-enzyme-inducing antibiotics and hormonal contraceptives. BMJ Evid-Based Med. 2021;26(3):112-113.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





