US Pharm. 2023;48(3):40-44.
ABSTRACT: Multiple sclerosis (MS), a chronic, unpredictable, and debilitating autoimmune disease of the central nervous system, can significantly impact patients’ quality of life. Symptoms vary widely and range from mild to severe. Disease-modifying therapy (DMT), including monoclonal antibody (mAb) treatment, is the standard of care for MS because these agents slow the disease progression while reducing the frequency and severity of relapses. In MS, mAbs modulate the immune system to slow effects on the brain and spinal cord, and they are generally preferred because of their efficacy, favorable adverse-effect profiles, and dosing frequency. Recent research has focused on the development of novel therapies. Pharmacists can play various roles in MS management and mAb use, and their involvement will continue to grow as new therapies become available.
Multiple sclerosis (MS) is a chronic, unpredictable autoimmune disease of the central nervous system (CNS) in which T cells attack the spinal cord and brain while leaving the muscles and nerves intact. This attack leads to inflammation and eventual demyelination (i.e., loss of the myelin sheath protecting nerve fibers), as well as axonal injury or loss within the CNS.1-4 Areas where demyelination occurs are commonly referred to as plaques or lesions; these appear on MRI, leading to a diagnosis of MS.2 Once a nerve becomes demyelinated, its electrical conduction slows and weakens, leading to symptoms of MS.1,3 There is no cure for MS.
Symptoms
Symptoms of MS vary widely and range from mild to severe in nature. As MS progresses, symptoms typically worsen due to increased axonal injury and other complex manifestations of the disease.3 These symptoms can be managed with pharmacotherapy, but many treatment challenges exist. Common early symptoms of MS include fatigue, weakness, tingling, numbness, and blurred vision; common late symptoms include cognitive deterioration, muscle spasms, pain, incontinence, depression, sensitivity to heat, sexual dysfunction, difficulty walking (gait instability), and severe vision disturbances.3,4
Clinical Subtypes
MS is a progressive disease that worsens over time. The four main subtypes of MS are clinically isolated syndrome (CIS), relapsing-remitting multiple sclerosis (RRMS), secondary progressive multiple sclerosis (SPMS), and primary progressive multiple sclerosis (PPMS); SPMS may be further categorized as active or nonactive.5,6 CIS, which is the term for a patient’s first episode of neurologic symptoms, is typically acute and lasts at least 24 hours. Fever and infection are absent during this first episode, but the patient experiences neurologic symptoms. A diagnosis of MS is made based on the detection of brain lesions by MRI. The presence of lesions signifies that the likelihood of another episode or relapse is high.3,4 A relapse involves new neurologic symptoms lasting at least 24 to 48 hours that arise from a new injury in the brain.3 To determine whether a patient’s MS is progressing, it is important to determine whether a symptom is new or recurring.
RRMS, the most common subtype, is generally the most mild. In RRMS, patients have new or worsened neurologic symptoms including those listed in the previous section. These relapses are followed by periods of incomplete or complete recovery known as remissions. During remission, the symptoms may disappear or become more stable and permanent.3,5 Further progression of MS does not occur during this remission period. In SPMS, which often follows RRMS, the patient experiences further worsening of neurologic function and progression of disability.4-6 The active form of SPMS involves relapses, new MRI activity showing additional plaque formation during a set period, or both; these features do not occur with the nonactive form.5,6 PPMS differs from the other subtypes in that early relapses and remissions are absent, but neurologic function worsens from the onset of MS symptoms.5 The treatment strategy depends on the MS subtype; therefore, it is important to correctly distinguish between the different subtypes.
Monoclonal Antibodies
Disease-modifying therapy (DMT) is the standard of care for patients with MS. The primary function of DMT is to alter the course of the disease. In MS, the overall goals of DMT are to lessen relapses and lesions and to delay physical and mental disability; however, these medications do not cure MS or relieve symptoms, so they do not help patients feel better or improve their quality of life. Other therapies are required for symptom management; these may include medications for cognitive impairment, depression, fatigue, gait impairment, spasticity, tremors, seizures, bladder dysfunction, and erectile dysfunction.2-4 Monoclonal antibodies (mAbs) are considered a DMT. An mAb is a protein produced in a laboratory that attaches to a specific target within the body, such as an antigen, particular type of cell, or virus.7 Common uses of mAbs include the treatment of COVID-19 infection, cancer, transplant rejection, autoimmune disorders, and nervous-system disorders. In MS, mAbs are used to modulate the immune system to lessen CNS attacks and effectively reduce relapses as well as the inflammation that results in lesions.2-4
In 2004, the FDA approved natalizumab for relapsing forms of MS, making it the first mAb approved for this indication. Since then, alemtuzumab and ofatumumab have been approved for RRMS and ocrelizumab has been approved for progressive forms of MS. Additionally, rituximab is used off-label for RRMS and—like the other mAbs—is recommended by the American Academy of Neurology (AAN) as a potential DMT option.8
In 2018, the AAN updated its practice guideline regarding DMT in adults with MS.8 In general, mAbs should be prescribed based on clinical judgment, and initiation should be tailored to the patient after discussing goals of therapy and obtaining a detailed history. If one mAb is not a good fit for the patient, the guideline suggests that the prescriber evaluate disease activity, adherence, side-effect profile, and specific mechanisms of each therapy before switching. For initiating DMT therapy, the AAN suggests alemtuzumab or natalizumab for patients in the relapsing-remitting phase who have highly active MS. Ocrelizumab is recommended for patients with PPMS, as it is the only DMT shown to affect disease progression in patients who are ambulatory. Ocrelizumab should be used in patients who are likely to benefit unless risks outweigh the benefit.8 TABLE 1 summarizes the mAbs used in MS, including their indications, adult dosing recommendations, adverse effects, monitoring parameters, and other important information.
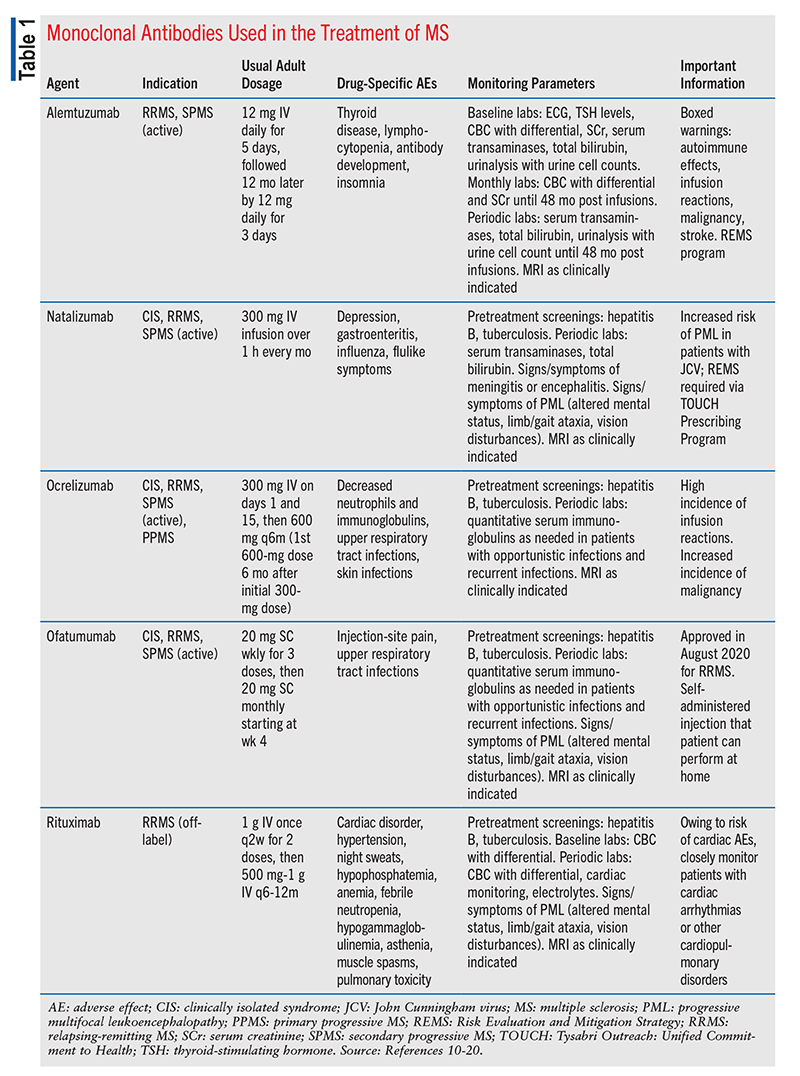
When natalizumab is being considered, patients should be screened via a simple blood test for the John Cunningham virus (JCV) antibody. If the result is positive, the patient is at increased risk for developing progressive multifocal leukoencephalopathy (PML), a condition involving the white matter of the brain.8,9 PML is caused by polyomavirus JC (i.e., JCV). This virus is harmless in most people, but in those positive for the JCV antibody, it can be reactivated through mAb therapy. This reactivation can be severe in that the virus will attack the cells that make myelin. The mortality rate of PML is around 30% to 50%, and patients who survive may be left with severe neurologic disabilities.9 The risk of PML increases depending on the level of anti-JCV antibody response and whether the patient has been on prior immunosuppression.9 The AAN suggests using another mAb if the risk is high, although ocrelizumab and rituximab also carry this risk.8
Pretreatment Medications
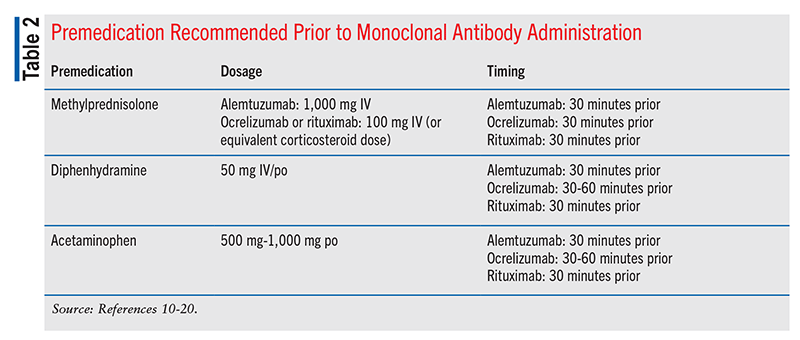
Drug-specific adverse effects of mAbs are listed in TABLE 1; however, for all mAbs, the most common adverse effects include fever, chills, fatigue, headache, hypotension, dermatologic reactions, nausea, vomiting, and diarrhea. Given the IV-infusion administration of most mAbs, many of these adverse effects occur during a hypersensitivity-related infusion reaction.10-20 To reduce the frequency and severity of these reactions, patients are commonly given medications and fluids prior to an infusion. Common premedications administered prior to IV infusion include methylprednisolone to prevent immune-mediated reactions, diphenhydramine for pruritus and dermatologic reactions, and acetaminophen to prevent fever and chills. TABLE 2 summarizes premedication recommendations for alemtuzumab, ocrelizumab, and rituximab when given via IV infusion, along with dose and optimal timing. It should be noted that natalizumab is also administered via IV infusion, but it does not have specific premedication recommendations.10-20
Recommended Vaccines
Because mAbs interfere with a patient’s immune system, there is an increased risk of upper respiratory tract infections, lower respiratory tract infections, urinary tract infections, fungal infections, and viral infections.11-21 Vaccine-preventable infections can significantly impact morbidity, mortality, and quality of life in patients with MS. Patients with MS who are receiving DMT are at risk for severe vaccine-preventable infections, specifically varicella zoster virus and hepatitis B virus. The AAN has a separate guideline specifically on vaccines in MS that advises against the use of live vaccines in patients using mAbs because of their weakened immune system; live vaccines should be administered 6 weeks prior to therapy initiation, if possible. Inactivated vaccines, including the influenza vaccine, are safe to administer and are strongly encouraged in order to prevent disease unless a patient-specific contraindication exists. However, if the patient is experiencing a relapse, all vaccines should be postponed until clinical resolution, based on data suggesting that immunizations can worsen relapse severity.21
Therapies in the Pipeline
The prognosis of MS has changed significantly since the development of DMT, specifically mAbs. Several therapy options are currently undergoing clinical trials as either adjunctive therapy or monotherapy in patients with MS. At the end of 2021, 11 phase III clinical trials were evaluating the use of four novel drugs for the treatment of relapsing and remitting forms of MS: IMU-838, ubituximab, tolebrutinib, and evobrutinib.22
Although these drugs offer hope, perhaps the most promising advance is a vaccine for MS prevention. For more than 40 years, the epidemiology of MS has been attributed to infectious mononucleosis, which is caused by the Epstein-Barr virus (EBV).22,23 Research published in 2022 validated the link between EBV and MS based on an analysis of 10 million U.S. service members conducted over the course of a decade.24 The researchers concluded that the patients who were exposed to EBV were approximately 32 times more likely to develop MS than those who were not, with only one patient developing MS without prior EBV exposure. Although most patients with MS had prior EBV exposure, not all patients with EBV exposure developed MS. Factors that increased the risk of MS in these patients included cigarette smoking, vitamin D deficiency, and genetics. The researchers concluded that if EBV infection could be prevented, the risk of MS could be reduced.24 Several biotechnology companies have made significant progress in advancing vaccines specifically for EBV prevention.23
The Pharmacist’s Role
Pharmacists remain an accessible resource for patients, caregivers, and providers. As clinical trials continue and evidence-based guidelines are updated, pharmacists should keep abreast of current guidance on the use of mAbs in MS. Pharmacists working in specialty pharmacy or clinic settings are often heavily involved in the monitoring of MS therapy in order to ensure efficacy and safety. Additionally, pharmacists in any setting may assist providers with prior authorizations or appeals necessary for obtaining insurance coverage and help patients enroll in assistance programs offered by drug manufacturers. Pharmacists can educate and counsel patients on medications and what to expect from mAb therapy. Pharmacists can also reinforce the goals of therapy and improve adherence to the medication by following up with the patient on a regular basis. Barriers to adherence can be discussed during each interaction, and the pharmacist can aid in solutions. Pharmacists can play a variety of roles in MS management and the use of mAbs, and their involvement will continue to grow as new therapies become available.
REFERENCES
1. National Institute of Neurological Disorders and Stroke (NINDS). Multiple sclerosis. www.ninds.nih.gov/health-information/disorders/multiple-sclerosis. Accessed September 13, 2022.
2. Huang WJ, Chen WW, Zhang X. Multiple sclerosis: pathology, diagnosis and treatments. Exp Ther Med. 2017;13(6):3163-3166.
3. Olek MJ, Howard J. Clinical presentation, course, and prognosis of multiple sclerosis in adults. Post TW, ed. UpToDate. Waltham, MA: UptoDate, Inc. www.uptodate.com. Accessed September 11, 2022.
4. Bainbridge JL, Miravalle A, Shieen Wong P, Makelky MJ. Multiple sclerosis. In: DiPiro JT, Yee GC, Posey LM, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 11th ed. New York, NY: McGraw Hill; 2020.
5. National Multiple Sclerosis Society. Types of multiple sclerosis. www.nationalmssociety.org/What-is-MS/Types-of-MS. Accessed October 3, 2022.
6. My-MS.org. Types of multiple sclerosis. https://my-ms.org/ms_types.htm. Accessed October 12, 2022.
7. National Cancer Institute. Monoclonal antibody. www.cancer.gov/publications/dictionaries/cancer-terms/def/monoclonal-antibody. Accessed September 19, 2022.
8. Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777-788.
9. NINDS. Progressive multifocal leukoencephalopathy. www.ninds.nih.gov/health-information/disorders/progressive-multifocal-leukoencephalopathy. Accessed October 12, 2022.
10. Olek MJ, Mowry E. Disease-modifying therapies for multiple sclerosis: pharmacology, administration, and adverse effects. Post TW, ed. UpToDate. Waltham, MA: UptoDate, Inc; 2022. www.uptodate.com. Accessed September 20, 2022.
11. Alemtuzumab. In: Lexi-Drugs (Lexicomp Online). Waltham, MA: UpToDate, Inc; February 18, 2022. https://online.lexi.com. Accessed September 29, 2022.
12. Lemtrada (alemtuzumab) product information. Cambridge, MA: Genzyme Corp; August 2022.
13. Natalizumab. In: Lexi-Drugs (Lexicomp Online). Waltham, MA: UpToDate, Inc; February 18, 2022. https://online.lexi.com. Accessed September 21, 2022.
14. Tysabri (natalizumab) product information. Cambridge, MA: Biogen Inc; December 2021.
15. Ocrelizumab. In: Lexi-Drugs (Lexicomp Online). Waltham, MA: UpToDate, Inc; February 18, 2022. https://online.lexi.com. Accessed September 21, 2022.
16. Ocrevus (ocrelizumab) product information. South San Francisco, CA: Genentech, Inc; August 2022.
17. Ofatumumab. In: Lexi-Drugs (Lexicomp Online). Waltham, MA: UpToDate, Inc; February 18, 2022. https://online.lexi.com. Accessed September 21, 2022.
18. Kesimpta (ofatumumab) product information. East Hanover, NJ: Novartis Pharmaceuticals Corp; September 2022.
19. Rituximab. In: Lexi-Drugs (Lexicomp Online). Waltham, MA: UpToDate, Inc; February 18, 2022. https://online.lexi.com. Accessed October 3, 2022.
20. Rituxan (rituximab) product information. South San Francisco, CA: Genentech, Inc; December 2021.
21. Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594.
22. Hoffman M. Pipeline update: status of clinical development for multiple sclerosis. Neurology Live. www.neurologylive.com/view/pipeline-update-status-clinical-development-multiple-sclerosis. Accessed September 16, 2022.
23. Maple PA, Ascherio A, Cohen JI, et al. The potential for EBV vaccines to prevent multiple sclerosis. Front Neurol. 2022;13:887794.
24. Boyle A. Ground-breaking study in U.S. military finds link between MS, Epstein-Barr virus. U.S. Medicine. www.usmedicine.com/clinical-topics/multiple-sclerosis/ground-breaking-study-in-u-s-military-finds-link-between-ms-epstein-barr-virus/. Accessed September 18, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






