US Pharm.
2006;7:80-88.
As
the incidence of hypertension rises in concert with the obesity epidemic,
pharmacists need to be prepared to counsel their hypertensive patients seeking
relief from cold symptoms. First-line therapy for the common cold includes
rest, adequate fluid intake, humidification for expectoration, and avoidance
of others to minimize viral transmission.1 However, beyond OTC pain
relievers, decongestants are generally the pharmacologic agents of choice for
congestion associated with the common cold. Decongestants are sympathomimetic
agents that act primarily on alpha-adrenergic receptors, with some activity on
beta-adrenergic receptors.2 The alpha agonist activity causes
vasoconstriction of the superficial blood vessels in the nasal mucosa,
reducing edema, nasal congestion, and tissue hyperemia, and increasing nasal
patency.2 Decongestants not only cause constriction of nasal
vessels; their systemic action is associated with insomnia, nervousness,
tremor, urinary retention, loss of appetite, and cardiovascular side effects
including increase in blood pressure, tachycardia, and palpitations.1,2
Therefore, the FDA requires that the following warning be placed on both oral
and topical decongestants: "Do not use this product if you have heart disease,
high blood pressure, thyroid disease, diabetes, or difficulty in urination due
to enlargement of the prostate gland, unless directed by a doctor."3
This article will focus on
standards of care and medications used for nasal congestion, including oral
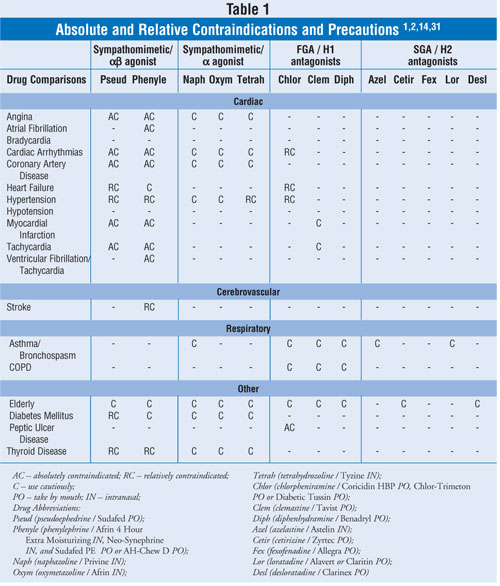
and topical nasal decongestants and alternatives to decongestants. Table 1
lists conservative, pooled information concerning absolute and relative
contraindications and precautions for the agents discussed in this review.
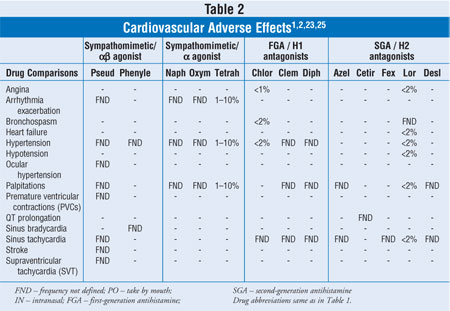
Table 2 provides limited, pooled, cardiovascular (CV) adverse event (AE)
frequencies.
DECONGESTANTS
Oral Decongestants
Pseudoephedrine:
Evaluative trials regarding oral decongestant use in hypertensive patients are
quite limited. Recently Salerno et al.4 performed a meta-analysis
(MA) of some pertinent available pseudoephedrine studies in an attempt to
provide more conclusive information regarding the safety of these products in
hypertensive patients. This MA included 24 studies with 1,285 patients and 45
total treatment arms. Thirty-one treatment arms used immediate-release (IR)
formulations and 14 treatment arms used sustained-release (SR) formulations.
Seven of the 45 arms investigated patients with treated, stable hypertension,
and five arms investigated pseudoephedrine's effects on the normal BP
elevation during exercise.4
Overall, there was a
statistically significant 1-mmHg increase in systolic blood pressure (SBP) but
no difference in diastolic blood pressure (DBP). Heart rate (HR) increased by
approximately 3 beats per minute (bpm). Also, longer study durations were
associated with a less pronounced effect on SBP. However, there was no such
association with regard to DBP or HR.4
In the 31 IR treatment arms,
there was a statistically significant 1.5-mmHg increase in SBP but no increase
in DBP. HR increased by 2 bpm. There was a dose-response relationship for SBP,
DBP, and HR.4 In the 14 SR arms, there was a statistically
significant 4-to-5–bpm HR increase but no detected systolic or DBP difference.
When data from only controlled
hypertensive patients (BP <140/90 mmHg) were analyzed, a statistically
significant 1-mmHg increase in SBP was detected but no difference was found
for DBP or HR. None of the five trials that included exercise testing revealed
any statistically significant differences in SBP, DBP, or HR.4
Although the authors reported
no clinically significant AEs, there were two patients whose mean arterial
pressure (MAP) increased by 20 mmHg and there were 30 reported episodes of
loss of BP control. MAP is calculated by multiplying DBP by 2 + SBP and then
dividing this value by 3. DBP is counted twice as much as SBP because diastole
accounts for two thirds of the cardiac cycle. Unfortunately, baseline MAP and
BP were not provided.4
The authors concluded,
"Pseudoephedrine modestly increases SBP and HR, with the greatest effects seen
in IR formulations, higher doses, and shorter-term medication administration.
Patients with stable, controlled hypertension do not seem to be at higher risk
for BP elevation than other groups when given pseudoephedrine along with their
antihypertensive medications."4 However, the authors noted BP
elevations greater than 140/90 mmHg in 3% of patients, so "the risk-benefit
ratio should be evaluated carefully before using sympathomimetic agents in
at-risk individuals."4
Limitations of this MA
included a relatively small evaluable sample size (n=1,260), inconsistent
baseline BP data, low numbers of elderly patients, and inadequate information
regarding confounding medications and/or conditions. Also, the authors pointed
out that their result may have "overestimated the effect of pseudoephedrine"
since the higher-quality studies of this MA "showed less pronounced effects on
vital signs."4 Furthermore, none of the trials within this MA
contained patients with uncontrolled hypertension.4-6
Phenylephrine (AH-Chew D,
Sudafed PE)
CV safety data are
lacking, thus limiting the ability to make a recommendation for or against
using this agent in controlled, hypertensive patients.
Topical Decongestants
Requiring FDA Warning
The FDA mandates
that topical decongestants include the same warning as stated for oral
decongestants.1,7,8 Communications received by the FDA have argued
that systemic distribution of topical decongestants is so small as to have no
effect on BP and HR.8 However, "the FDA examined the studies
submitted by the correspondents and failed to find support for the assertion
that topical products would be safe for patients with high blood pressure or
heart disease."8 The FDA also found that "cardiovascular
adverse reactions are among the most frequent AEs with topical nasal
decongestants, exceeded only by rebound congestion," which generally occurs
with more than 3–5 days of consistent use.8 The FDA concluded
that "all sprays and drops produced bradycardia, tachycardia, hypertension,
and hypotension."8 This appears to be more of a problem with
oxymetazoline than with phenylephrine.8 However, phenylephrine has
a much shorter duration of action, dosed every 4 hours compared to
oxymetazoline's twice daily dosing recommendation.3,7
There are four case reports of
CV adverse effects that warrant special mention. The first was in a
73-year-old male with a past medical history (PMH) of cerebellar degeneration
and peripheral neuropathy who experienced bradycardia, hypotension, and
syncope after using oxymetazoline nasal spray. This was attributed to a
baroreceptor reflex impairment.9 The second case was in a
35-year-old male who experienced an ischemic stroke after using oxymetazoline
nasal spray every 3 days for 20 years.10 The third case was of a
31-year-old female with a PMH including hiatal hernia, cigarette smoking, and
remote marijuana use who experienced a thunderclap headache 20 minutes after
using oxymetazoline. This patient had been using 2 to 3 sprays twice daily on
a consistent basis. (A thunderclap headache has a sudden, severe onset and
often happens before a severe intracranial vascular incident.) The headache
resolved after discontinuation of oxymetazoline.11 The last case
warranting mention involved a 44-year-old male who had a thalamic hemorrhage
with temporary left hemiparesis one day after naphazoline use. His
BP was 190/120 mmHg upon presentation. He was discharged home on day 8 without
the need for any BP medications. All motor deficits recovered.12
Topical Decongestants Not
Requiring an FDA Warning
Levmetamfetamine
(Vicks Inhaler) and propylhexedrine (Benzedrex) are two OTC nasal
decongestants that are not mandated by the FDA to carry the warning. However,
their roles are limited due to lack of comparable efficacy data relative to
other sympathomimetic decongestants, limited duration of action, and abuse
potential, including reports of medication extraction from the inhaler for
intravenous and/or oral abuse.1 Although levmetamfetamine is
generally safe and effective for OTC use, propylhexedrine appears to cause
headache, hypertension, nervousness, and tachycardia.1
Various topical rubs and vapor
agents containing menthols, camphor, and/or eucalyptus oil appear to be
somewhat effective for improving congestion symptoms associated with the
common cold.13 Topical rubs can be applied to the chest and/or
throat, and vapor agents can be added to warm or hot vaporizers. As with
topical levmetamfetamine and propylhexedrine, data regarding comparable
efficacy relative to more traditional topical and oral nasal decongestants are
lacking. However, if patients are not hypersensitive to the components of
these agents, they can be beneficial in relieving nasal congestion and are
safe for use in hypertensive patients.13,14
Throat lozenges containing
menthol appear to be no more effective than placebo lozenges when evaluated
objectively; however, there are data supporting subjective efficacy in
patients experiencing congestion symptoms from the common cold.14-16
ALTERNATIVES TO
DECONGESTANTS
Oral Antihistamines
Antihistamines are
commonly used alternatives to decongestants. Although these agents have a
negligible effect on congestion, they generally have a moderate effect on
runny nose and a pronounced effect on sneezing and watery eyes, which also
occur with the common cold.17,18 Most of the data supporting these
benefits were obtained from studies using first-generation antihistamines
(FGAs).
Neither FGAs nor
second-generation antihistamines (SGAs) adversely affect BP. Therefore, these
agents may be used to help decrease runny nose in hypertensive patients who
have no comorbidities. However, not all antihistamines are devoid of adverse
cardiac effects, and in practice, we rarely treat patients with hypertension
alone. Therefore, information regarding non-BP associated CV adverse effects
follows.
First-Generation
Antihistamines
Cardiotoxicity is
more likely with FGAs than with SGAs.17,19 The quinidine-like local
anesthetic and anticholinergic properties appear to be responsible for the
observed adverse cardiac effects, including tachycardia, electrocardiogram
(ECG) changes, hypotension, and arrhythmias. "Although the relative risk of
cardiotoxicity with these drugs is real (patients taking the drugs have an
increased risk), the absolute risk is small (occurs in only a small number of
people even when a large number of people take the drug). However, OTC FGAs
have been shown to be associated with a higher rate of ventricular arrhythmias
than the SGA terfenadine," withdrawn from the U.S. market due to its
life-threatening QT interval prolonging effects.17 Also,
cardiotoxicity is more likely with higher doses. Although cardiovascular
effects are uncommon, FGAs should be used conservatively in patients with
cardiac disease.2
Second-Generation
Antihistamines
Based on current
data, SGAs appear to pose a lower risk for drug interactions and cardiac side
effects than FGAs.20 However, CV effects are variable among the
SGAs. It is important to consider agent-specific data and reports.
• Loratadine
There has been one
case report of torsades de pointesand QT interval prolongation when loratadine
was combined with amiodarone.21 This occurred in a 73-year-old
female with a history of hypertension, hyperlipidemia, paroxysmal atrial
fibrillation, and left ventricular hypertrophy (LVH) with diastolic
dysfunction who was admitted to the hospital for syncope. She was taking
chronic amiodarone 200 mg daily for atrial fibrillation. Other medications
included cilazapril, pravastatin, and warfarin. She had been given loratadine
10 mg daily "a few days prior to admission … for a suspected allergic
reaction."21 The authors of this report suggested "prior to
prescribing loratadine concomitantly with a drug that may potentially prolong
the QT interval, an ECG should be done and repeated several hours after
ingestion of the first dose."21 If "an increase in QT
interval or dispersion is noted, loratadine should be discontinued and rhythm
monitoring initiated."21
There is some evidence of a
statistically significant QT interval prolongation when loratadine 20 mg daily
and nefazodone are used concomitantly. This interactive AE appears to be
correlated with increased concentrations of loratadine.20 According
to the World Health Organization Collaborating Centre for International Drug
Monitoring at Uppsala, Sweden, there have been 57 reports of ventricular
arrhythmias associated with loratadine. Twenty-seven of these reports did not
mention other confounding or interacting drugs, and five of these patients
died.22
• Desloratadine (Clarinex)
Desloratadine is the active
metabolite of loratadine. Although there have been reports of spontaneous
adverse effects such as tachycardia and palpitations as listed in the product
package insert, it does not appear that this agent causes QT interval
prolongation.23 Even when "administered alone in a higher dose or
in combination with ketoconazole or erythromycin, no prolongation of the QT
interval was observed."20
• Fexofenadine (Allegra)
Fexofenadine is a noncardiotoxic
water-soluble metabolite of terfenadine. "As far as its cardiological safety
is concerned, fexofenadine has shown an excellent CV profile in clinical
trials."24 "No statistically significant increase in mean QT
interval compared to placebo was observed in 714 seasonal allergic rhinitis
patients given fexofenadine … in doses of 60 to 240 mg twice daily for two
weeks."25 A separate study of 432 patients receiving 180 mg
for 14 days to three months supports this data.25
There is one reported case of
a 67-year-old male with hypertension and mild LVH who had QT interval
prolongation after taking 180 mg daily for two months.25 Although
there was a temporal relationship between fexofenadine use and QT interval
prolongation, there were several possible confounding contributors to the
arrhythmia. This patient's age, history of hypertension, and recent withdrawal
of antihypertensive therapy would be expected to increase his risk for QT
interval prolongation and ventricular dysrhythmia. In addition, no continuous
ECG monitoring was conducted. Given all of these limitations, the authors
indicated it would be "unfair to draw conclusions on the basis of a single
case report."25
• Cetirizine (Zyrtec)
Cetirizine is the active metabolite
of the sedating antihistamine hydroxyzine. At recommended doses, cetirizine
has not caused QT interval prolongation.19,26 Current data
including pooled reports indicate that adverse CV events including cardiac
failure, hypertension, palpitation, and tachycardia would be expected to occur
in less than 2% of patients.26
Topical Antihistamines
Azelastine
(Astelin/Optivar)
Astelin is the intranasal topical
formulation and Optivar is the ophthalmic topical formulation of azelastine.
This agent does not appear to cause an increase in CV adverse event risk
relative to placebo.1,14
Miscellaneous Options
Saline Mist and Humidification
An isotonic saline
mist is very safe and soothing for a dry and irritated nose. Humidification
can also help loosen congestion and facilitate mucociliary clearance and
expectoration.1 Evaporative or steam humidifiers appear to be
preferred over cool mist humidifiers because the latter may be more likely to
disseminate aerosols contaminated with allergens."27 However,
all humidifiers must be cleaned regularly per manufacturer recommendations to
minimize risk of exposure to contaminants, i.e., bacteria, protozoa or fungi.
27-29
External Dilators
Breathe Right Nasal Strips are
external nasal dilators worn over the bridge of the nose. As the
cross-sectional area of the nasal valve determines nasal airway resistance,
these strips open the nasal airway by applying approximately 25 grams of
outward pulling force through two parallel plastic springs. A small,
randomized controlled trial showed external dilators significantly increase
the size of the nasal valve area and decrease the level of congestion in
normal subjects.30 As would be expected, symptoms recur after
removal. The obvious major benefit of this option is no increased risk of CV
adverse effects.
Conclusion
While pharmacists
often answer questions concerning products for relief of common cold symptoms,
selecting appropriate products for the patient with hypertension is a
challenge. Unfortunately, there is no one product that can be recommended to
provide safe and effective relief of nasal congestion in all patients with
high blood pressure. In addition, such patients typically have comorbidities
that also must be considered when choosing therapy. The information presented
in this review will assist pharmacists in making safe and effective
therapeutic recommendations for nasal congestion in their hypertensive
patients.
References
1. Micromedex Web site. Available
at: www.thomsonhc.com/home/dispatch (accessed November 13, 2005).
2. Clinical Pharmacology. Available
at: cpip.gsm.com or cp.gsm.com/ (accessed November 13, 2005).
3. Code of Federal Regulations. Drugs
for human use. Title 21, Volume 5, Chapter 1, subchapter D, revised as of
April 1, 2005. Available at:
www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=341.80
(accessed January 6, 2006).
4. Salerno SM, Jackson JL, Berbano
EP. Effect of oral pseudoephedrine on blood pressure and heart rate: a
meta-analysis. Arch Intern Med. 2005;165:1686-694.
5. Chua SS, Benrimoj SI, et al. A
controlled clinical trial on the cardiovascular effects of single doses of
pseudoephedrine in hypertensive patients. Br J Clin Pharmacol.
1989;28:369-72.
6. Coates ML, Rembold CM, et al. Does
pseudoephedrine increase blood pressure in patients with controlled
hypertension? J Fam Pract. 1995;40:22-26.
7. Cold, cough, allergy,
bronchodilator, and antiasthmatic drug products for over-the-counter human
use. Federal Register Part 341:235-52. Available at:
www.fda.gov/cder/otcmonographs/Allergy/Cold,Cough,Allergy(341).pdf (accessed
January 24, 2006)
8. Pray SW. Blood pressure effects of
nasal decongestants. U.S. Pharm. Available at:
www.uspharmacist.com/oldformat.asp?url=newlook/files/cons/feb00cyp.htm.
(accessed January 6, 2006).
9. Glazener F, Blake K, Gradman M.
Bradycardia, hypotension, and near-syncope associated with Afrin
(oxymetazoline) nasal spray. N Engl J Med. 1983;309:731.
10. Montalban J, Ibanez L, et al.
Cerebral infarction after excessive use of nasal decongestants. J Neurol
Neurosurg Psychiatry. 1989;52:541-543.
11. Loewen AHS, Hudon ME, Hill MD.
Thunderclap headache and reversible segmental cerebral vasoconstriction
associated with use of oxymetazoline nasal spray. Can Med Assoc J.
2004;171:593-594.
12. Zavala JAA, Pereira ER, et al.
Hemorrhagic stroke after naphazoline exposition. Arch Neuropsychiatr.
2004;62:889-891.
13. Cohen BM, Dressler WE. Acute
aromatics inhalation modifies the airways. Effects of the common cold.
Respiration. 1982;43:285-293.
14. eFacts. Available at
www.factsandcomparisons.com (accessed Nov. 15, 2005).
15. Eccles R, Jawad MS, Morris S. The
effects of oral administration of (-)-menthol on nasal resistance to airflow
and nasal sensation of airflow in subjects suffering from nasal congestion
associated with the common cold. J Pharm Pharmacol. 1990
Sep;42(9):652-654.
16. Eccles R, Morris S, Jawad MS. The
effects of menthol on reaction time and nasal sensation of airflow in subjects
suffering from the common cold. Clin Otolaryngol Allied Sci. 1990;39-42.
17. The Antihistamine Impairment
Roundtable. First do no harm: managing antihistamine impairment in patients
with allergic rhinitis. J Allergy Clin Immunol. 2003;111:5:S835-S842.
18. Health care guideline: rhinitis.
Institute for Clinical Systems Improvement. May 2003. Available at
www.icsi.org/display_file.asp?FileId=147&title=Chronic%20Rhinitis (pages 10
and 25 accessed January 9, 2006)
19. Chandler, C. Drug class
review on second-generation antihistamines: final report. November 2004.
Available at www.oregon.gov/DAS/OHPPR/HRC/docs/AH_EPC.pdf (accessed
January 6, 2006).
20. Paakkari, I. Cardiotoxicity of
new antihistamines and cisapride. Toxicol Lett. 2002;127:279-284.
21. Atar S, Freedberg NA, et al.
Torsades de pointes and QT prolongation due to a combination of loratadine and
amiodarone. Pacing Clin Electrophysiol. 2003;26:785-786.
22. Clark S. Dangers of nonsedating
antihistamines. Lancet. 1997;349:1268.
23. Clarinex package insert. Available at
www.spfiles.com/piclarinex.pdf (accessed November 12, 2005).
24. Allegra package insert. Available
at products.sanofi-aventis.us/allegra/allegra.pdf (accessed November 12, 2005).
25. Dhar S, Hazra PK, et al.
Fexofenadine-induced QT prolongation: a myth or fact? Br J Dermatol
. 2000;142:1260.
26. Zyrtec prescribing information.
Available at www.pfizer.com/pfizer/download/uspi_zyrtec.pdf (accessed November
12, 2005).
27. Arundel AV, Sterling EM, Biggin
JH, Sterling TD. Indirect health effects of relative humidity in indoor
environments. Environ Health Perspect. 1986;65:351-361.
28. Assendelft AV, Forsen KO,
Keskinen H, Alanko K. Humidifier-associated extrinsic allergic alveolitis.
Scand J Work Environ Health. 1979;5:35-41.
29. Park JH, Spiegelman DL, et al.
Predictors of airborne endotoxin in the home. Environ Health Perspect.
2001;109:859-864.
30. Latte J, Taverner D. Opening the
nasal valve with external dilators reduces congestive symptoms in normal
subjects. Am J Rhinol. 2005;19:215-219.
31. Lexi-Comp. Lexi-Drugs (Comp +
Specialties). Available at www.lexi.com (accessed November 15, 2005.
To comment on this article, contact
editor@uspharmacist.com.






