US Pharm. 2019:44(11)HS-2-HS-7.
ABSTRACT: Dementia with Lewy bodies (DLB) is the second most common form of neurodegenerative dementia in older adults. To be diagnosed, patients will present with dementia in addition to at least two of the following core symptoms: fluctuating cognition, recurrent visual hallucinations, REM sleep behavior disorder, and/or parkinsonism. Recommended treatment strategies focus on providing symptomatic relief; there is no FDA-approved medication to treat DLB. Pharmacists can help prevent hospitalizations and resultant morbidity by ensuring that patients do not take medications known to exacerbate fall risk and worsen cognitive deficits.
Broadly defined, neurodegenerative dementia is a progressive cognitive decline that adversely and markedly impairs the patient’s overall functionality.1 Dementia with Lewy bodies (DLB) accounts for only 10% to 20% of total dementia diagnoses, but its economic and societal costs are nonetheless burdensome.2 Patients with DLB incur higher healthcare costs, have longer hospitalizations, report lower quality of life, and have caregivers with higher levels of distress when compared with patients with Alzheimer disease (AD).3,4 There is no FDA-approved medication to halt the progression of cognitive decline in DLB, which may progress more rapidly than in other dementias.4 Current treatment strategies that focus on symptomatic control provide modest benefit.3 This article will explain the pathology of DLB and examine current evidence-based treatment recommendations.
PATHOLOGY
The theorized pathology behind DLB shares characteristics with that of other dementias, albeit with a few notable exceptions. In all dementias, an interaction takes place between neurons and inflammatory markers, leading to an accumulation of protein aggregates, but the type and deposition locales of these proteins differ among the types of dementia.5-7 Beta-amyloid plaques and alpha-synuclein aggregates maintain the proper structure of the neuron’s shape in nonpathogenic neurons. When amyloid precursor proteins are cleaved by beta-secretase enzymes instead of by alpha-secretase, a large and insoluble beta-amyloid protein is released.8 Beta-amyloid then aggregates and interacts with Tau proteins, making it no longer able to provide the microtubule backbone of the neuron. Rather, Tau creates large plaques with beta-amyloid, which become the hallmark tangles and plaques of AD and DLB.5-9
In DLB, alpha-synuclein’s role is similar to that of beta-amyloid and Tau. If alpha-synuclein is processed incorrectly by enzymes, it can be cleaved in a way that makes it clump together, forming what we call Lewy bodies.6,7 These Lewy bodies go on to damage neurons and result in wide-scale brain loss.6 Postmortem analyses show that Lewy bodies deposit in parts of the brainstem, basal limbic system, and neocortical structures, which explains the clinical presentation of DLB in a patient.5,10
CLINICAL PRESENTATION
As with all types of dementia, progressive cognitive decline is one of the first noticeable clinical features of DLB; however, the quality of cognitive impairments seen in the early stages of DLB is different than that of other dementias.1 Memory recall remains intact until later stages, but attention, executive function, and visuospatial skills are the first to decline in DLB. This deterioration in upper-level cognitive functioning is likely due to degradation of the neocortical areas, specifically the frontal cortex.5 These deficits lead to sudden difficulty when driving and when completing tasks with multiple steps.11 In addition to noticeable cognitive decline, other core features evident upon presentation include fluctuating cognitive impairment, recurrent visual hallucinations, parkinsonism, and REM sleep behavior disorder (RBD).2
Sixty percent to 80% of patients exhibit fluctuating cognition in the early stages of DLB. Episodes of staring into space, daytime drowsiness, and disorganized speech are examples of these spontaneous, waxing and waning changes in behavior and consciousness that are a core clinical feature of DLB.2 Lasting anywhere from minutes to hours, fluctuations are variable in presentation and severity, which may contribute to the frequent misdiagnosis of DLB.11 The severity of fluctuations and attention deficits are prognostic markers and may signal a worse course of illness.11
A second core feature of DLB is the emergence of visual hallucinations, which will often occur concomitantly with cognitive fluctuations. They are possibly caused by high concentrations of Lewy body deposition in the amygdala, hippocampus, and other temporal lobe regions.10 Eighty percent of patients report seeing things that other people do not; the visions can be complex, detailed images of people, animals, or children, or they can be unformed shapes seen in the periphery.2 Auditory hallucinations and delusions have also been reported. The neuropsychiatric symptoms of DLB are burdensome to caregivers and are often the impetus behind placement in a residential care facility.11
While not a requirement for diagnosis, spontaneous motor disturbances such as bradykinesia, rest tremor, and/or rigidity (parkinsonism) occur in approximately half of patients during the early stage of DLB, but 25% of patients never exhibit them.2 Lewy bodies begin accumulating in the brainstem, a location that is known to dictate motor movements and is highly damaged in Parkinson disease (PD).10 The onset of parkinsonism in relation to the onset of dementia is of diagnostic significance: When associated with DLB, parkinsonism occurs at the same time or after the onset of dementia.11
DIAGNOSIS
As indicated in Table 1, patients must present with dementia plus at least two of the following core symptoms to be diagnosed with DLB: fluctuating cognition, recurrent visual hallucinations, RBD, or parkinsonism.2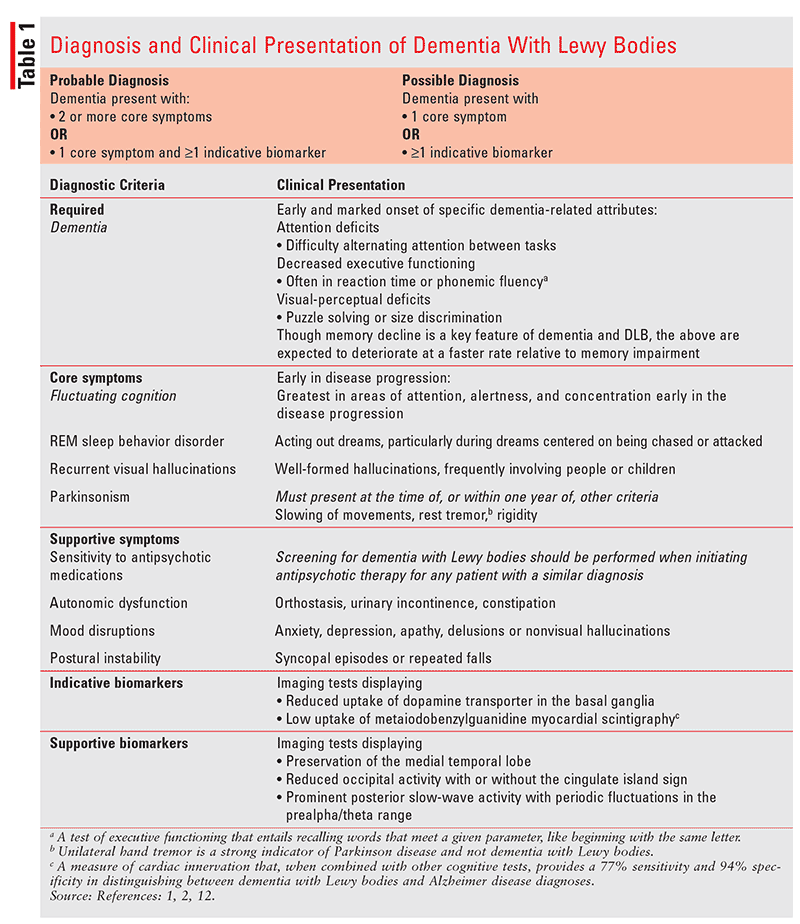
Given the overlap in DLB pathology with AD and PD, clues like these may help a caregiver or clinician recognize a diagnosis that could otherwise be overlooked. Pharmacists are encouraged to be vigilant when consulting with patients about newly prescribed medications and to also look for signs of sensory loss, hallucinations, bilateral tremor, ataxia, and REM sleep disturbances, among the others discussed here, to assist in the correct diagnosis of DLB.5,10,12
TREATMENT
It is hypothesized that an extensive loss of cholinergic neurons is responsible for the cognitive decline and visual hallucinations that occur early and substantially in DLB; thus, administering agents that prevent breakdown of acetylcholine provides symptomatic relief.3 The cholinesterase inhibitors (ChEIs) donepezil (Aricept) and rivastigmine (Exelon) are recommended as first-line agents for treating DLB.2 Pooled data from a recent meta-analysis showed that treatment with ChEIs improved cognitive function, motor function, and behavioral symptoms, and some improvements were seen in attention and language.13 ChEIs appear to be relatively safe3; only rivastigmine had a higher incidence of worsening parkinsonism when compared with placebo (relative risk 1.18, P = .0001), whereas donepezil did not.13 Memantine, an N-methyl d-aspartate receptor antagonist, and zonisamide, an antiepileptic, also have evidence supporting their use.
Donepezil
A 12-week, phase II exploratory study found that donepezil at 5 and 10 mg/day was superior to placebo in treating cognitive decline, which was assessed by changes from baseline in Mini Mental Status Examination (MMSE) scores. Mean difference in MMSE scores at endpoint was -0.4 for placebo; donepezil 5 mg/day was 3.8 and 10 mg/day was 2.4, both with P <.001. Behavioral improvements were assessed using the Neuropsychiatric Inventory (NPI), which measures domains such as delusions, hallucinations, cognitive fluctuations, and anxiety. Both 5 and 10 mg/day showed statistically significant improvements in behavior from baseline, but only 10 mg/day conferred a reduction in caregiver burden (P = .004). Safety measures were similar across all groups when compared with placebo.14
An extension analysis confirmed a sustained benefit on cognition in the 10 mg/day group up to 52 weeks (2.4, P <.001), but not in the 5 mg group (1.3, P = .018). All groups, including placebo, showed a reduction in behavioral symptoms; thus, it cannot be determined that the benefit was solely due to the study drug. Subjects receiving 10 mg/day had a slightly higher incidence of mild gastrointestinal complaints and parkinsonism, suggesting that long-term administration of donepezil is safe and well-tolerated.15 It is recommended that patients be monitored for decrease in appetite and gradual weight loss.14
Furthermore, a confirmatory phase III trial showed improvement in cognitive function only in the 10 mg/day group when compared with placebo (change in MMSE scores from baseline to 12 weeks in 10 mg: 2.2; placebo: 1.6, P = .0016); no benefit in treating behavioral symptoms was seen with either dose. Safety data were consistent with previous randomized, controlled trials (RCTs).16
Rivastigmine
Rivastigmine’s utility in DLB is less studied than donepezil’s; however, data suggest that it is an efficacious treatment option for behavioral symptoms such as anxiety, visual hallucinations, and delusions.3
The first RCT to evaluate rivastigmine in DLB followed subjects given 6-12 mg/day of study drug or placebo over 20 weeks. Rivastigmine’s effects on behavioral symptoms (as measured by changes in NPI) were statistically and clinically significant; in fact, over half of the subjects reported that their delusions and hallucinations were almost absent during the study period, only to quickly return during the 3-week washout/discontinuation phase. Also reported were less anxiety and diminished apathy as compared with placebo. The trial failed to show any significant differences in mean MMSE scores when compared with placebo (rivastigmine: 1.5; placebo: -0.1, P = .072).17
Twenty-nine of the patients who completed the aforementioned RCT were recruited for an open-label study lasting 96 weeks. Despite major methodological issues, such as lacking a control group and being open-label, the study noted improvements in MMSE scores up to week 24 and in NPI scores up to Week 12.18
Treatment-emergent tremor and gastrointestinal distress were notably increased during both of the studies, but were likely due to the rapid dose-titration schedule in which subjects reached the highest dose by Week 8. A slower dose escalation is recommended to increase tolerability. Other reported side effects include hypersalivation and urinary frequency.17
Memantine
Although evidence is lacking that it helps with cognition, memantine (Aricept) has data supporting its efficacy in treating behavioral symptoms and RBD.3 An RCT examining the efficacy and safety of memantine in a population including DLB and Parkinson disease dementia (PDD) patients found greater improvement in behavioral symptoms via reductions in NPI scores with memantine 20 mg/day as opposed to placebo (P = .041). There were no differences in adverse events among the groups, though other studies have indicated the possibility of worsening neuropsychiatric symptoms.19
A potential niche for memantine that warrants further research is its efficacy in treating RBD. A small study of 42 patients showed that 20 mg/day of memantine significantly decreased physical movement during sleep compared with placebo at 24 weeks (P = .006).20
Zonisamide
One of the therapeutic challenges of treating parkinsonism in DLB is the possibility of exacerbating psychotic symptoms as a result. Levodopa, a dopaminergic agent, is a highly efficacious treatment for the motor symptoms in PD, but reports estimate that only 30% of DLB patients experience any benefit, possibly because of dose-limiting adverse events such as psychosis.11,21 Using small doses and slow upward titration according to clinical response may prevent adverse events.5 Zonisimide (Zonegran), a second-generation antiepileptic drug that is thought to enhance dopamine release, may overcome this challenge.22
One phase II RCT demonstrated that zonisamide used adjunctively with levodopa significantly improved parkinsonism without worsening hallucinations or cognitive function. One hundred fifty-eight patients were randomized to receive zonisamide 25 or 50 mg/day or placebo for 12 weeks. Only the 50 mg/day group saw improvement in motor symptoms when compared with placebo (P = .003). The incidence of adverse events (somnolence, decreased weight, and appetite) was highest in the 50-mg group (65.3%) compared with placebo (50%), but the intensity of these adverse events was not greater as the dose increased. Although more studies are needed, preliminary data regarding the efficacy of adjunctive zonisamide are encouraging.22
ROLE OF THE PHARMACIST
Preventing avoidable hospitalizations in DLB patients is of paramount importance; not only is the length of stay longer and more costly, but a single hospitalization is known to worsen overall functioning beyond what would be attributed to advancing pathology and to increase risk of death in dementia patients post-discharge.4,23
The two most common causes for hospitalization in DLB are potentially modifiable. In fact, a retrospective study found that 40% of hospitalizations were due to an exacerbation of neuropsychiatric disturbances and 24% were due to falls.4 Another pertinent finding of the study was that 41% of these hospitalized patients took antipsychotic medications at home, despite the known risks of their use causing worsening cognitive function and increasing mortality in all dementia patients. Using home antipsychotics (excluding clozapine or quetiapine) correlated with a higher level of care post-discharge (odds ratio, 2.02).4
It is well within the pharmacist’s purview to monitor for the use of potentially inappropriate medications in patients with DLB. As seen in Table 2, medications such as anticholinergics, antipsychotics, and benzodiazepines can worsen constipation, urinary retention, cognitive deficits, confusion, and dizziness.1 Patients also may develop life-threatening sensitivity reactions from first-generation antipsychotics; as such, their use is highly discouraged.4 Medications with alpha-receptor blocking properties (antihypertensives and prostate treatments) can exacerbate fall risk.1 By reviewing patient records for medications with these properties, pharmacists can play a direct role in preventing a hospitalization and perhaps even prolonging a life. 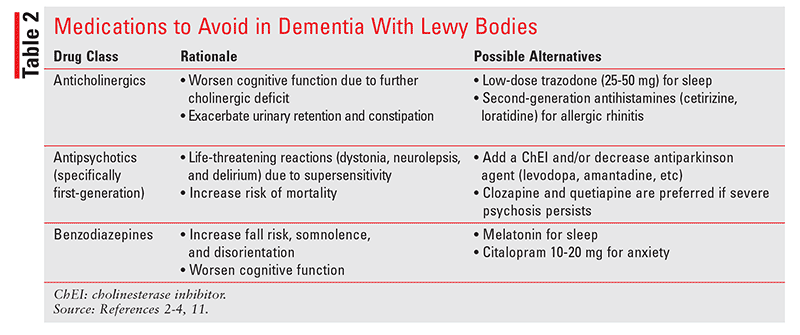
CONCLUSION
DLB is a neurodegenerative condition for which there is currently no cure. Because they have higher healthcare costs, shorter time from diagnosis to death, and higher rates of institutionalization, patients with DLB may have a poorer prognosis than those with AD.1 Treatment with ChEIs (donepezil and rivastigmine) and memantine may improve cognitive function, visual hallucinations, and behavioral symptoms; however, refractory neuropsychiatric symptoms and repeated falls often lead to hospitalization and subsequent transfers to higher levels of care. Pharmacists can help increase quality of life in these patients by monitoring for medications that increase fall risk and questioning the unnecessary use of antipsychotics.3,4
REFERENCES
1. Mueller C, Ballard C, Corbett A, Aarsland D. The prognosis of dementia with Lewy bodies. Lancet Neurol. 2017;16:390-398.
2. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100.
3. Palermo G, Ceravolo R, Bonuccelli U. Advances in the pharmacotherapeutic management of dementia with Lewy bodies. Expert Opin Pharmacother. 2018;19:1643-1653.
4. Spears CC, Besherat A, Monari EH, et al. Causes and outcomes of hospitalization in Lewy body dementia: A retrospective cohort study. Parkinsonism Relat Disord. 2019;64(7):106-111.
5. Mrak RE, Griffin WS. Dementia with Lewy bodies: definition, diagnosis, and pathogenic relationship to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3(5):619-625.
6. Marsh SE, Blurton-Jones M. Examining the mechanisms that link -amyloid and -synuclein pathologies. Alzheimers Res Ther. 2012;4(2):11.
7. Garcia-Esparcia P, López-González I, Grau-Rivera O, et al. Dementia with Lewy bodies: molecular pathology in the frontal cortex in typical and rapidly progressive forms. Front Neurol. 2017;8:89.
8. Murphy MP, LeVine H 3rd. Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19(1):311-323.
9. Chung EJ, Babulal GM, Monsell SE, et al. Clinical features of Alzheimer disease with and without Lewy bodies. JAMA Neurol. 2015;72(7):789-796.
10. Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002;125(Pt 2):391-403.
11. Chin KS, Teodorczuk A, Watson R. Dementia with Lewy bodies: challenges in the diagnosis and management. Aust N Z J Psychiatry. 2019;53:291-303.
12. Ransmayr G. Dementia with Lewy bodies: prevalence, clinical spectrum and natural history. J Neural Transm Suppl. 2000;(60):303-314.
13. Meng YH, Weng PP, Song YX, Wang JH. Cholinesterase inhibitors and memantine for Parkinson’s disease dementia and Lewy body dementia: a meta-analysis. Exp Ther Med. 2019;17:1611-1624.
14. Mori E, Ikeda M, Kosaka K, et al. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled trial. Ann Neurol. 2012;72:41–52.
15. Mori E, Ikeda M, Nagai R et al. Long-term donepezil use for dementia with Lewy bodies: results from an open-label extension of phase III trial. Alzheimers Res Ther. 2015;7:5.
16. Ikeda M, Mori E, Matsuo K. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled, confirmatory phase III trial. Alzheimers Res Ther. 2015;7:4.
17. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo controlled international study. Lancet. 2000;356:2031-2036.
18. Grace J, Daniels S, Stevens T. et al. Long-term use of rivastigmine in patients with dementia with Lewy bodies: an open-label trial. Int Psychogeriatr. 2001;13(2):199-205.
19. Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:969-977.
20. Larsson V, Aarsland D, Ballard C, et al. The effect of memantine on sleep behaviour in dementia with Lewy bodies and Parkinson’s disease dementia. Int J Geriatr Psychiatry. 2010;25:1030-1038.
21. Molloy S, McKeith IG, O’Brien JT, Burn DJ. Role of levodopa in management of dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2005;76:1200-1203.
22. Murata M, Odawara T, Hasegawa K, et al. Adjunct zonisamide to levodopa for DLB parkinsonism: a randomized double-blind phase 2 study. Neurology. 2018;90:e664-e672.
23. Goodman RA, Lochner KA, Thambisetty M, et al. Prevalence of dementia subtypes in U.S. Medicare fee-for-service beneficiaries, 2011-2013. Alzheimers Dement. 2017;13(1):28-37.
To comment on this article, contact rdavidson@uspharmacist.com.






