US Pharm. 2023;48(5):8-12.
ABSTRACT: Obsessive-compulsive disorder (OCD) is a debilitating neuropsychiatric condition affecting approximately 2% of the world population. An obsession is an intrusive and persistent thought, image, or urge, whereas a compulsion is a ritual performed to suppress anxiety associated with an obsession. Most people with OCD have a comorbid condition, making diagnosis and treatment difficult. In addition to cognitive-behavioral therapy, pharmacologic agents including selective serotonin reuptake inhibitors and clomipramine are approved for OCD. Not all patients respond to current pharmacotherapies for OCD, and some patients who do may still have residual symptoms, so alternative strategies such as therapy augmentation are important. Pharmacists can play a pivotal role in OCD education, counseling, and prevention of adverse drug reactions and drug-drug interactions.
Obsessive-compulsive disorder (OCD), a debilitating neuropsychiatric condition, affects 1% to 2% of the world population.1 Most people with OCD have a comorbid condition (e.g., depression, eating disorder, anxiety disorder), making diagnosis and treatment difficult. As its name denotes, OCD is characterized by the presence of both obsessions and compulsions. An obsession is an intrusive and persistent thought, urge, or image that is distressing (e.g., fear of causing harm, fear of germs, need for order, feelings of doubt) and frequently results in avoidance behavior, whereas a compulsion is a ritual (e.g., excessive hand washing, counting, arranging) performed to suppress anxiety associated with an obsession.2 Obsessions and compulsions usually involve >1 hour per day, causing significant mental distress and interfering with the performance of important daily tasks.
The age of onset for OCD follows a bimodal pattern, peaking in early adolescence and then early adulthood (age 20-29 years). The exact cause of OCD is unknown, but it is believed that 40% to 60% cases are heritable.2 Altered function in certain brain areas and cognitive deficit in performing higher-level tasks (e.g., executive functions) have also been reported. Dysfunctions in serotonin, dopamine, and glutamate pathways have been identified in OCD.3
Most adults with OCD are aware of their symptoms and want to control this distressing behavior. With appropriate treatment, patients can achieve symptom relief and remission. Treatment options for OCD include cognitive-behavioral therapy and selective serotonin reuptake inhibitors (SSRIs), usually used together.2 This article will focus on current pharmacotherapy options for this condition and augmentation strategies for OCD that is resistant to pharmacotherapy.
Current Pharmacotherapy
SSRIs (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline) and clomipramine are currently used to treat patients with OCD. In a meta-analysis of 17 clinical trials involving 3,097 participants, SSRIs were found to be more effective than placebo in reducing symptoms of OCD, and efficacy did not differ between individual SSRIs.4 However, a patient may respond to a certain agent and not others.
In a systematic review and network meta-analysis, the tricyclic antidepressant (TCA) clomipramine demonstrated efficacy similar to that of SSRIs for treating OCD symptoms.5 Because of its unfavorable adverse-effect (AE) profile (discussed below), clomipramine has been designated a second-line treatment option for OCD patients who do not respond to SSRIs.2 One study found that although higher doses of SSRIs were more efficacious than low or moderate doses, they were associated with a significantly higher proportion of dropouts due to the AE burden.6 TABLE 1 lists the starting and target dosing for pharmacologic agents used to treat OCD.2 The maximum tolerated SSRI dose should be used for ≥8 to 12 weeks, with the patient’s response assessed after at least 4 to 6 weeks on the maximum tolerated dose.7,8
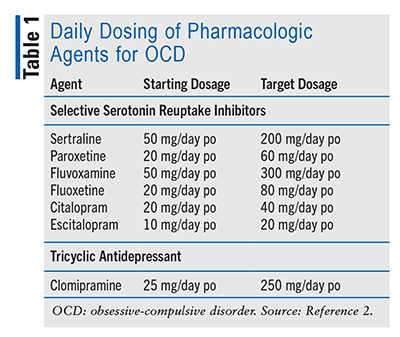
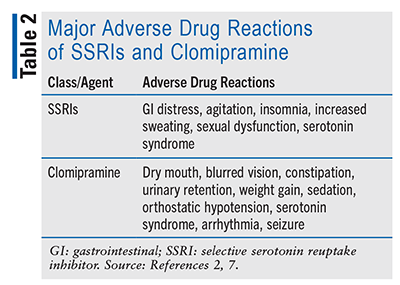
SSRIs selectively inhibit neuronal serotonin transporter (SERT), preventing reuptake of serotonin from the synaptic cleft and leading to prolonged neurotransmission.9 The most common AEs associated with SSRIs are gastrointestinal distress, agitation, insomnia, increased sweating, and sexual dysfunction.2 The daily dose of citalopram should not exceed 40 mg (20 mg in certain circumstances—e.g., hepatic impairment, age >60 years, CYP2C19 inhibitor coadministration) because of the increased risk of QT prolongation.2 The utilization of SSRIs or clomipramine is associated with an increased risk of suicidal thoughts and behavior in young adults (ages ≤24 years) within the first few months of treatment or when the dosage is changed.9 Therefore, constant monitoring is required for these patients as well as patients of other ages.9
Clomipramine, a tertiary amine TCA, acts predominantly by inhibiting SERT and resulting in prolonged neurotransmission.9 It also blocks norepinephrine uptake and blocks muscarinic cholinergic receptors, alpha-1 adrenergic receptors, H1 histaminergic receptors, and voltage-gated sodium channels in the heart and central nervous system (CNS).7 Clomipramine-induced anticholinergic effects include dry mouth, blurred vision, constipation, and urinary retention. Histaminergic blockade may result in sedation and weight gain. Alpha-receptor blockade may cause orthostatic hypotension, whereas sodium-channel blockade may precipitate cardiac arrhythmia and seizure. Clomipramine dosed at >200 mg/day may increase the risk of cardiac and CNS events, so serum concentrations must be monitored.2 The use of clomipramine or SSRIs increases the risk of serotonin syndrome whether used alone or concomitantly with another agent that increases serotonin activity (e.g., triptans, TCAs, fentanyl, tramadol, monoamine oxidase inhibitors, linezolid); therefore patients, particularly if elderly, should be monitored for serotonin syndrome.9
If discontinued abruptly, SSRIs or clomipramine may cause withdrawal syndrome, which is characterized by dizziness, nausea/vomiting, paresthesia, headache, insomnia, agitation, and myoclonic jerking.9 These agents should therefore be tapered off slowly.9 TABLE 2 lists the major adverse drug reactions associated with SSRIs and clomipramine.
Treatment Resistance
In clinical trials, a 25% to 35% improvement in Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score signifies response to therapy.2 Not all patients respond to current pharmacotherapies for OCD, and some patients who do respond may continue to have residual symptoms. Factors contributing to treatment resistance include severity and onset of disease; inappropriate diagnosis; inappropriate medications; adverse drug reactions; compliance problems; and comorbid conditions.1
Augmentation Approaches
When a patient does not get adequate relief from an SSRI, viable approaches include switching to another SSRI or clomipramine or adding an augmentation agent. Antipsychotics (usually atypical antipsychotics) are used to augment the effects of approved OCD drugs in treatment-resistant patients.2
A meta-analysis of 14 randomized, double-blind, placebo-controlled trials (491 participants in total) concluded that antipsychotic agents may be used for augmentation with SSRIs.10 Risperidone, aripiprazole, and haloperidol had superior effects compared with placebo in SSRI-treated patients, and antipsychotics were effective for treating both obsessions and compulsions.10 It must be noted that these agents have not been FDA-approved for augmentation purposes.
TABLE 3 lists the atypical antipsychotics and their dosing for augmentation.1,7 These agents block dopaminergic D2 receptors and act as inverse agonists at 5HT2A receptors; aripiprazole is a partial agonist at D2 receptors.11 Reported AEs for these agents are weight gain, elevated glucose, and changes in lipid concentration.11

Clinical Trials
For more than 2 decades, no new drugs have been developed for the specific treatment of OCD symptoms.1 As it is currently believed that in addition to serotonin other neuronal pathways (e.g., dopaminergic) are involved in OCD pathology, medications from different therapeutic categories have been evaluated as augmentation agents in several clinical trials.1,3
Antidepressants: One study compared the efficacy and AE profiles of the serotonin-norepinephrine reuptake inhibitor venlafaxine with SSRI paroxetine in 150 OCD patients.12 In this 12-week trial, 75 patients received venlafaxine 300 mg/day and 75 patients received paroxetine 60 mg/day. There was no significant difference in therapeutic response between venlafaxine and paroxetine. The most common AEs for venlafaxine were somnolence, insomnia, dry mouth, and sweating; those for paroxetine were somnolence, headache, sweating, and nausea.12
In another trial, 61 patients receiving sertraline at an average dosage of 250 mg/day were randomly assigned to a daily dose of mirtazapine 39.5 mg or placebo.13 Of the 45 patients who completed the trial (mirtazapine, n = 22; placebo, n = 23), nine mirtazapine patients versus one placebo patient had a ≥35% decrease in Y-BOCS score.13
Antiepileptic Agents: In a 12-week double-blind study, topiramate was administered to treatment-resistant OCD patients who had been on their maximum tolerated SSRI dose for ≥12 weeks (and on the current dose for ≥6 weeks).14 Topiramate intervention resulted in improvements in the Y-BOCS compulsion subscale but not the obsession subscale. Because of AEs, the topiramate endpoint dose (dose range, 50-400 mg daily) was not well tolerated.14 In a different double-blind trial, patients on their current OCD therapy who received topiramate (mean dosage 180 mg/day) reported a 32% decrease in Y-BOCS score versus 2% of the placebo group.15
One study revealed that when added to serotonin reuptake inhibitor therapy in OCD patients, lamotrigine 100 mg/day for 16 weeks significantly improved compulsions, obsessions, and affective symptoms as well as total Y-BOCS score.16 Reported AEs from lamotrigine included sedation, fatigue, headache, and skin rashes.16
In a double-blind, placebo-controlled trial, treatment-refractory OCD patients were randomized to receive pregabalin or placebo plus current sertraline therapy.17 At the end of 12 weeks, 57% of pregabalin patients demonstrated a >35% decline in Y-BOCS versus 7% in placebo patients. Pregabalin was well tolerated by the study participants.17
Neuroprotective Agents: A randomized, double-blind, crossover, placebo-controlled trial evaluated 20 patients for OCD severity and psychosocial functions at baseline, after 2 weeks of tolcapone, and then after 2 weeks of placebo.18 There was a 1-week washout period between the tolcapone and placebo phases. It was found that the 2 weeks of tolcapone therapy significantly improved OCD symptoms versus placebo.18
In another study, fluvoxamine-treated OCD patients with a Y-BOCS score ≥21 received memantine or placebo for 8 weeks.19 For the first 4 weeks, memantine was administered at 10 mg/day, after which the dosage was increased to 20 mg/day. Upon study completion, 89% of memantine-treated patients (n = 17) versus 32% of placebo patients (n = 6) were in remission.19
One trial investigated memantine treatment in 32 patients who were resistant to SSRI therapy for OCD.20 Patients received either memantine 20 mg/day or placebo and were evaluated at baseline and every 4 weeks thereafter for 12 weeks. Compared with placebo, total Y-BOCS score was significantly reduced at the 8-week and 12-week evaluations. At 12 weeks, 73% of memantine patients had achieved treatment response.20
In a small study, 13 patients whose OCD was resistant to standard medication were given a glutamate inhibitor, riluzole, dosed at 50 mg twice daily.21 Riluzole was well tolerated, and more than one-half of patients had a >35% improvement in Y-BOCS score.21 However, in a different study using the same dosage, riluzole was only nominally beneficial versus placebo.22
Miscellaneous Agents: A double-blind, placebo-controlled trial investigated adjuvant celecoxib use over 8 weeks in 50 treatment-resistant OCD patients on 20 mg fluoxetine daily.23 Twenty-five patients took celecoxib 400 mg/day (200 mg twice daily); the remaining patients received placebo. Celecoxib augmentation significantly decreased obsessive and compulsive symptoms compared with placebo.23
A different 8-week study assessed ondansetron for augmentation in 44 patients with OCD.24 All patients received fluvoxamine (100 mg/day) for the first 4 weeks and 200 mg/day for the remaining 4 weeks, and they were randomized to either ondansetron 8 mg/day or placebo for all 8 weeks. Patients were evaluated with Y-BOCS and an AE checklist at baseline and then at 2, 4, 6, and 8 weeks. Ondansetron intervention significantly reduced total Y-BOCS score and obsession and compulsion subscores, with no difference in AE profile between ondansetron and control groups.24
In a placebo-controlled crossover study, drug-free OCD patients received two 40-minute infusions (one saline; the other ketamine 0.5 mg/kg).25 Patients showed improvement during the infusion period. One week post infusion, the ketamine group demonstrated a residual beneficial effect versus the placebo group.25
A meta-analysis of four clinical trials and five case reports found that N-acetyl cysteine, a glutamate modulator, at a dosage of 2,400 to 3,000 mg/day for a typically 12-week duration, decreased the intensity of OCD symptoms and had good tolerability and better AE profile.26
Minocycline is an immunomodulatory antibiotic. In a randomized, double-blind study, 102 patients were given fluvoxamine and either minocycline 100 mg twice daily or placebo for 10 weeks.27 Minocycline improved OCD scores significantly, and its AEs did not differ markedly from those for placebo.27
In a small trial, OCD patients who were unresponsive to paroxetine received pindolol 2.5 mg thrice daily or placebo for 6 weeks.28 Pindolol treatment demonstrated a significant improvement in Y-BOCS score compared with placebo.28
One study randomized 24 OCD patients in a 1:1 ratio to receive either the glutamate receptor agonist glycine at a maximum of 60 g/day or placebo.29 Patients were evaluated at 4, 8, and 12 weeks. Only 14 patients completed the study because of poor palatability and nausea caused by the glycine. The study reported some improvements, but they were not statistically significant.29
Sarcosine, an endogenous antagonist of glycine transporter-1, increases glycine-mediated N-methyl-d-aspartate subtype glutamatergic neurotransmission. In one trial, a sarcosine dosage of 500 to 2,000 mg/day administered for 10 weeks resulted in a 17% reduction in Y-BOCS score.30
Methylphenidate use was examined in 44 SSRI-refractory OCD patients in an 8-week randomized, double-blind, placebo-controlled trial.31 One group received fluvoxamine 250 mg/day and extended-release methylphenidate; the other group received fluvoxamine 250 mg/day and placebo. Forty-one patients completed the trial. Compared with the placebo group, the methylphenidate group had statistically significant improvements in total Y-BOCS and obsession-subscale scores, and the cumulative response score was higher as well. Methylphenidate was well tolerated overall.31
The promising findings from the studies summarized above are preliminary, and therefore conclusions cannot be made as yet. Long-term, statistically robust multicenter trials are required to determine the utility of these agents for augmentation. Comprehension of the cellular and molecular mechanisms will provide the clinical rationale for these agents’ use in OCD therapy.
Conclusion
New therapeutic agents and effective augmentation strategies are needed to facilitate the treatment of this debilitating condition. This is particularly important for patients whose OCD is unresponsive to the current treatment options. Meanwhile, pharmacists can play a pivotal role in patient counseling and prevention of adverse drug reactions and drug-drug interactions. Pharmacists are also well positioned to educate physicians and other healthcare providers about appropriate therapeutic strategies for OCD.
REFERENCES
1. van Roessel PJ, Grassi G, Aboujaoude EN, et al. Treatment-resistant OCD: pharmacotherapies in adults. Compr Psychiatry. 2023;120:152352.
2. Hirschtritt ME, Bloch MH, Mathews CA. Obsessive-compulsive disorder: advances in diagnosis and treatment. JAMA. 2017;317(13):1358-1367.
3. Fenske JN, Petersen K. Obsessive-compulsive disorder: diagnosis and management. Am Fam Physician. 2015;92(10):896-903.
4. Soomro GM, Altman D, Rajagopal S, et al. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev. 2008;2008(1):CD001765.
5. Skapinakis P, Caldwell DM, Hollingworth W, et al. Pharmacological and psychotherapeutic interventions for management of obsessive-compulsive disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2016;3(8):730-739.
6. Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850-855.
7. Koran LM, Hanna GL, Hollander E, et al. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(suppl 7):5-53.
8. Koran LM, Simpson HB. Guideline Watch (March 2013): Practice Guideline for the Treatment of Patients With Obsessive-Compulsive Disorder. Washington, DC: American Psychiatric Association; 2013.
9. DeBattista C. Antidepressant agents. In: Katzung BG, Vanderah TW, eds. Basic and Clinical Pharmacology. 15th ed. New York, NY: McGraw-Hill; 2021:550-572.
10. Dold M, Aigner M, Lanzenberger R, Kasper S. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: an update meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2015;18(9):pyv047.
11. DeBattista C. Antipsychotic agents & lithium. In: Katzung BG, Vanderah TW, eds. Basic and Clinical Pharmacology. 15th ed. New York, NY: McGraw-Hill; 2021:529-549.
12. Denys D, van der Wee N, van Megen HJ, Westenberg HG. A double blind comparison of venlafaxine and paroxetine in obsessive-compulsive disorder. J Clin Psychopharmacol. 2003;23(6):568-575.
13. Mowla A, Baniasadipour H. Is mirtazapine augmentation effective for patients with obsessive-compulsive disorder who failed to respond to sertraline monotherapy? A placebo-controlled, double-blind, clinical trial. Int Clin Psychopharmacol. 2023;38(1):4-8.
14. Berlin HA, Koran LM, Jenike MA, et al. Double-blind, placebo-controlled trial of topiramate augmentation in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2011;72(5):716-721.
15. Mowla A, Khajeian AM, Sahraian A, et al. Topiramate augmentation in resistant OCD: a double-blind placebo-controlled clinical trial. CNS Spectr. 2010;15(11):613-617.
16. Bruno A, Micò U, Pandolfo G, et al. Lamotrigine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. J Psychopharmacol. 2012;26(11):1456-1462.
17. Mowla A, Ghaedsharaf M. Pregabalin augmentation for resistant obsessive-compulsive disorder: a double-blind placebo-controlled clinical trial. CNS Spectr. 2020;25(4):552-556.
18. Grant JE, Hook R, Valle S, et al. Tolcapone in obsessive-compulsive disorder: a randomized double-blind placebo-controlled crossover trial. Int Clin Psychopharmacol. 2021;36(5):225-229.
19. Ghaleiha A, Entezari N, Modabbernia A, et al. Memantine add-on in moderate to severe obsessive-compulsive disorder: randomized double-blind placebo-controlled study. J Psychiatr Res. 2013;47(2):175-180.
20. Modarresi A, Sayyah M, Razooghi S, et al. Memantine augmentation improves symptoms in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a randomized controlled trial. Pharmacopsychiatry. 2018;51(6):263-269.
21. Coric V, Taskiran S, Pittenger C, et al. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biol Psychiatry. 2005;58(5):424-428.
22. Pittenger C, Bloch MH, Wasylink S, et al. Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: a pilot randomized placebo-controlled trial. J Clin Psychiatry. 2015;76(8):1075-1084.
23. Sayyah M, Boostani H, Pakseresht S, Malayeri A. A preliminary randomized double-blind clinical trial on the efficacy of celecoxib as an adjunct in the treatment of obsessive-compulsive disorder. Psychiatry Res. 2011;189(3):403-406.
24. Heidari M, Zarei M, Hosseini SM, et al. Ondansetron or placebo in the augmentation of fluvoxamine response over 8 weeks in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2014;29(6):344-350.
25. Rodriguez CI, Kegeles LS, Levinson A, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38(12):2475-2483.
26. Oliver G, Dean O, Camfield D, et al. N-acetyl cysteine in the treatment of obsessive compulsive and related disorders: a systematic review. J Clin Psychopharmacol Neurosci. 2015;13(1):12-24.
27. Esalatmanesh S, Abrishami Z, Zeinoddini A, et al. Minocycline combination therapy with fluvoxamine in moderate-to-severe obsessive-compulsive disorder: a placebo-controlled, double-blind, randomized trial. Psychiatry Clin Neurosci. 2016;70(11):517-526.
28. Dannon PN, Sasson Y, Hirschmann S, et al. Pindolol augmentation in treatment-resistant obsessive-compulsive disorder: a double-blind placebo controlled trial. Eur Neuropsychopharmacol. 2000;10(3):165-169.
29. Greenberg WM, Benedict MM, Doerfer J, et al. Adjunctive glycine in the treatment of obsessive-compulsive disorder in adults. J Psychiatr Res. 2009;43(6):664-670.
30. Wu PL, Tang HS, Lane HY, et al. Sarcosine therapy for obsessive compulsive disorder: a prospective, open-label study. J Clin Psychopharmacol. 2011;31(3):369-374.
31. Zheng H, Jia F, Han H, et al. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: a randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019;29(3):397-404.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





