US Pharm. 2014;39(1):47-51.
ABSTRACT: Pharmacists are trained to recognize the most common adverse effects of drugs; however, any member of the medical team can easily overlook side effects that appear to be new medical conditions. Drug-induced neurologic conditions may result from single-agent drug regimens, but they are more likely to occur when multiple agents that confer risk are administered concomitantly. Educating patients about these risks and remaining vigilant in clinical investigations of unusual adverse effects are key elements to increasing the safety of drug management.
Along with awareness of the improved health outcomes resulting from drug therapy comes the recognition that drug therapy may also contribute to the emergence of new disorders. Mindful consideration of the possibility of drug-induced neurologic symptoms must be part of any ongoing evaluation of the evolving body of evidence obtained through clinical case reports. The emergence of new neurologic side effects of drugs heightens the challenges prescribers face when considering drug therapy. These side effects can result in potential misdiagnoses, including false psychiatric diagnoses, in the case of some drugs. Unexpected and unpredictable drug interactions can result in a confusing range of symptoms that may be identified as a new medical condition.
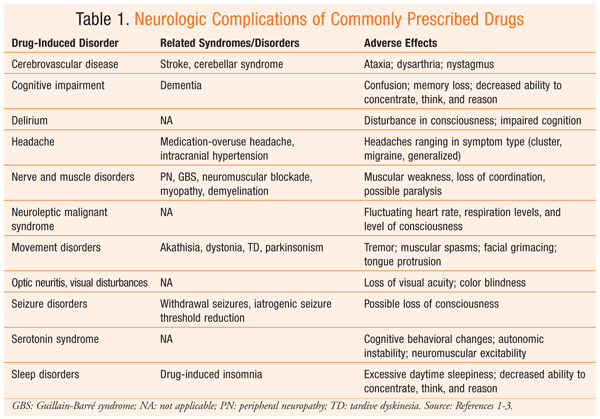
The term neurologic side effect is commonly used to describe a new drug-induced neurologic syndrome and/or disorder. Pharmacists may not routinely anticipate or recognize these new conditions caused by common drug agents. Changes in the central nervous system (brain, spinal cord) or peripheral nerves can cause a wide variety of symptoms, including loss of coordination and muscle strength, numbness, loss of consciousness, seizures, and paralysis (TABLE 1).1-3 In July 2013, the FDA added a black box warning to the antimalarial drug mefloquine about serious neurologic side effects, including dizziness, loss of balance, and tinnitus.4
Often, it is difficult to determine whether a neurologic condition is caused by a drug. A key factor to consider when reviewing clinical case reports and/or reporting through MedWatch is clear and consistent identification of the suspect drug, as well as a detailed report of the proposed drug-induced disorder. One of the more difficult aspects of such an investigation is ensuring the specificity of the event related to the drug by ruling out other potential causes of the disorder. Limits in premarketing data make postmarketing surveillance essential to identifying new neurologic side effects of drugs.
Cerebrovascular Effects
Cerebellar syndrome is a consequence of the disruption of normal function of the brain region that is responsible for coordination and balance. Drug-induced cerebellar syndrome can be caused by a number of drugs, including phenytoin, lithium, carbamazepine, certain chemotherapeutic agents, and aminoglycoside antibiotics. In addition to loss of coordination, some patients may experience dysarthria and nystagmus. Many cases are reversible; however, permanent cerebellar syndrome can result, especially with administration of high doses and concurrent use of agents conferring risk, such as lithium plus antipsychotics. Although its incidence is rare and based on case reports, cerebellar syndrome is included in current drug-drug interaction databases, with warnings to clinicians to use appropriate caution.2
Deep venous thrombosis, pulmonary embolism, myocardial infarction, and stroke have been reported with the use of estrogens and/or progestin therapy. While hormone replacement therapy is no longer routinely prescribed for menopausal women, the risk of neurologic side effects must be considered in women taking injectable hormone therapies or oral contraceptives.3
When agents that can have cerebrovascular effects are clinically indicated, the pharmacist must assist the patient in managing any modifiable risk factors, such as hypertension, diabetes, and hypercholesterolemia. Dietary changes, increased physical activity, and smoking cessation are critical nonpharmacologic interventions.
There have been reports of cerebrovascular and other vascular effects from antipsychotic agents. A significantly increased incidence of transient ischemic attack, cerebral ischemia, unspecified cerebrovascular disorders, and stroke has been reported versus placebo in patients older than 73 years who have dementia-related psychosis. Individual stroke risk factors such as smoking, hypertension, and diabetes increase the risk of this adverse neurologic event. Some of the second-generation antipsychotics (SGAs) are more likely to cause metabolic complications; thus, when considering the use of SGAs, a patient’s potential cumulative risk should be taken into account. Elderly patients with dementia-related psychosis being treated with antipsychotics are at increased risk for death secondary to stroke and other cerebrovascular complications.3
Cognitive Impairment and Delirium
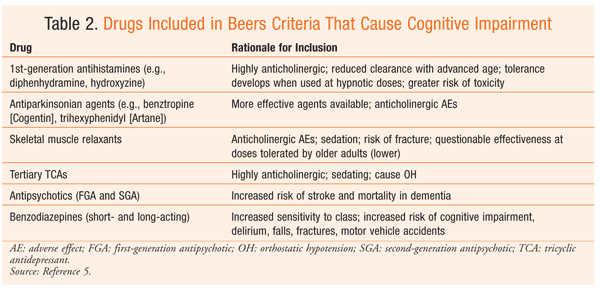
Delirium can be a neurologic side effect of drugs, especially in (although not limited to) elderly patients. Delirium is characteristically acute in onset and precipitates fluctuations in cognition, mood, attention, and arousal. Delirium can be reversed when the underlying causative agent is identified and removed. Adverse effects can cause subtle impairment of executive functions, thereby lessening the brain’s ability to regulate cognitive tasks such as working memory, problem-solving, and verbal reasoning. Increased awareness of the drugs that contribute to cognitive impairment is necessary, particularly with elderly patients.5
Prescribers can refer to the Beers Criteria to determine which drugs are linked to cognitive impairment (TABLE 2).5 Delirium and dementia are thought to originate with altered cholinergic transmission. Anticholinergic agents are among the classes with the highest risk of precipitating confusion and cognitive impairment, and the severity increases when multiple agents with confounding risks are used together. Research indicates that the total cumulative anticholinergic effect can better predict the development of delirium than the specific drug itself. Cognitive toxicity can develop quickly, especially when the patient is not educated about this potential drug-induced effect and worsens the risk by self-medicating with OTC drugs that increase the overall burden.5
Neuroleptic Malignant Syndrome
Neuroleptic malignant syndrome (NMS) is a drug-induced neurologic disorder caused by neuroleptic and antipsychotic drugs. Muscular rigidity, autonomic instability, fever, and changes in cognition (e.g., delirium) are hallmarks of NMS, which is thought to result from dopamine receptor D2 blockade in the corpus striatum, spinal cord, and hypothalamus. Although the incidence of NMS has declined since it was first reported, the potential for fatal outcome remains, particularly if providers fail to recognize the symptoms as a new neurologic syndrome and instead interpret them as deterioration of the primary mental health disorder. Once begun, NMS symptoms often progress rapidly, reaching peak severity at around 72 hours. The muscular rigidity and tremors often cause muscle-tissue breakdown and elevated creatine phosphokinase plasma concentrations.6
NMS symptoms are similar to those seen in serotonin syndrome (SS); thus, the differential diagnosis involves exclusion. Given the similarity of the conditions, obtaining a complete medication history and event timeline is critical to securing an accurate diagnosis. One distinguishing feature is the specific time course of development. SS typically develops within a few hours after exposure to the causative agent, whereas NMS tends to take longer—often several days—to emerge. Neuroleptics and antipsychotics can cause NMS when administered as single agents; however, the risk increases when they are given concurrently. Numerous drugs can cause NMS, including conventional first-generation antipsychotics (e.g., chlorpromazine and haloperidol) and other drugs with dopamine-antagonist activity (e.g., metoclopramide). Abrupt discontinuation of dopamine agonists such as levodopa can be problematic and may increase the risk of NMS.6
The development of NMS is unpredictable; however, the use of conventional antipsychotics with known risk, the concurrent use of multiple risk-bearing agents, and rapid dosage changes are prescribing practices that translate into an overall increased likelihood of developing this drug-induced neurologic disorder.3,6
Movement Disorders
Drug-induced movement disorders (DIMDs) continue to impose undue challenges on patients taking a variety of drugs. DIMDs can reduce quality of life, decrease drug adherence, and pose an increased risk of adverse outcomes owing to compromised performance of motor skills needed for activities of daily living. Drugs taken for psychiatric conditions, drugs for Parkinson’s disease, and gastrointestinal agents all contribute to the burden of drug-induced neurologic side effects. The drugs commonly implicated as causing DIMDs include dopamine-receptor blockers (antipsychotics) and antiemetics (e.g., metoclopramide). A number of drugs can cause tremor, which may be indistinguishable from idiopathic disease; however, pharmacists may be able to discern cases that are drug-induced by taking a careful history and evaluating all drugs in the regimen that are potentially causative. DIMDs can range from mild and distressing to permanent and disfiguring, so recognition of this adverse effect must occur as early as possible following the development of symptoms.1
It has been estimated that one-third to one-half of parkinsonism cases may be caused by drugs.2 While neuroleptic agents have been most widely implicated, antidepressants have also been identified in case reports involving extrapyramidal symptoms (EPS). Although parkinsonism is theorized to be associated mainly with dopamine imbalance in the nigrostriatal tract, the role of serotonin also is recognized. Serotonin receptors also affect dopamine release, with stimulation of the 5-hydroxytriptamine (5-HT)2A receptor inhibiting dopamine release and the 5-HT1A receptor stimulating its release. The newer atypical antipsychotics have been promoted as causing fewer EPS; however, these effects are still reported, and more commonly after patients have been exposed to the older agents that have greater EPS potential. SGAs with stronger dopamine blocking potential, such as risperidone, are most likely to cause EPS.
The range of movement disorders is vast. Akathisia is described as a feeling of restlessness coupled with an absolute need to move. While symptoms may improve at rest, forced restraint increases patient stress without reducing the core symptoms. Akathisia is generally early-onset and may be seen within the few first months of treatment. Tardive syndromes are abnormal involuntary movements that have a delayed onset, involve the tongue, face, and jaw, and may occur years after the drug is withdrawn. Tardive dyskinesia is a permanent and disfiguring condition, so patients must be educated about this risk and the need to immediately report any unusual movements of the face, lips, and/or tongue. Acute dystonia can occur with the first dose of an offending agent; the symptoms may be associated with distress and pain, as the patient may experience difficulty ambulating, breathing, and swallowing. Drugs used to treat some of these movement disorders include anticholinergic agents (e.g., benztropine and diphenhydramine) and benzodiazepines.2
Seizure Disorders
Drug-induced seizures may be difficult to differentiate from new-onset unprovoked seizure disorders associated with medical conditions. Seizures are thought to occur when nerve cells in the brain signal abnormally. These abnormal electrical firings may impair movements, actions, and/or level of consciousness. A patient who has two or more unprovoked seizures is considered to have epilepsy, which is estimated to affect 2.2 million people in the United States and 65 million people worldwide.7 Symptoms associated with drug-induced seizures are similar to those of non–drug-related seizures. The majority of seizures induced by drugs present as generalized tonic-clonic seizures.1
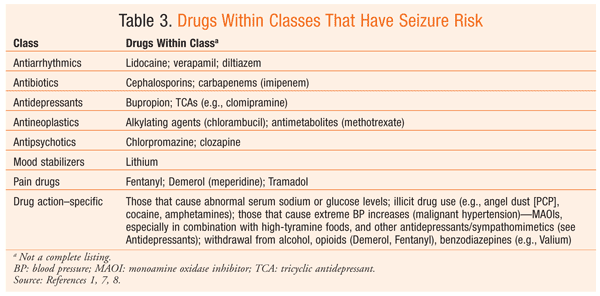
While certain drugs are used to control seizures, others can induce seizures in patients without a preexisting seizure disorder. Many substances can lower the seizure threshold (an individual’s unique balance between inhibition and excitation signals in the brain). The lower the threshold, the more likely a person is to experience a seizure. The risk of drug-induced seizure increases when multiple agents with potential risk are administered concomitantly. This is especially important when considering the impact of alcohol consumed along with a drug that lowers the seizure threshold. The withdrawal of drugs such as benzodiazepines also may provoke seizures. The risk of seizure varies among agents, but it can be reduced through proactive dosage reductions of the target drug and/or preventive dosing of antiepileptic agents when risky drug-regimen changes and/or combinations are indicated or otherwise unavoidable.
When investigating a possible drug-induced seizure, it is important to consider whether the suspected drug has been implicated in seizure activity at normal doses, or primarily in higher serum concentrations (as is the case with tricyclic antidepressants, theophylline, and clozapine). Supratherapeutic serum concentrations may be not a result of intended overdose, but rather an unpredicted drug interaction leading to an adverse outcome. Drug interactions may contribute to increased seizure activity in patients being treated for epilepsy when the antiepileptic regimen is compromised. Additionally, in considering the potential drug cause, pharmacists should be careful to note details about drug administration, such as initiation, dosage changes, and/or abrupt discontinuation. A number of patient factors further increase the risk of seizure precipitation, including age, metabolic abnormalities (e.g., electrolyte imbalance), and head trauma.7
Epilepsy is the fourth most common neurologic disorder in the U.S. after migraine, stroke, and Alzheimer disease. When patients with new-onset seizures are evaluated, drug history and current drug use should be evaluated to ensure that the seizures are not potentially caused by drugs.1 Although rare, seizure may occur with higher doses, in at-risk populations, and when administered with other seizure-inducing agents (TABLE 3).1,7,8
Serotonin Syndrome
SS, an acute iatrogenic drug-induced condition, is a constellation of predictable symptoms resulting from a serotonin excess and overstimulation of 5-HT receptors.2,6 Cognitive and behavioral changes, neuromuscular excitability, and autonomic instability occur. Patients generally present with sweating, agitation, tremor, fever, and nausea and vomiting. The presence of excess serotonin lessens the secretion of dopamine, and this inverse neurotransmitter relationship may explain the similarities seen in patients diagnosed with NMS. SS can range from mild to severe and is potentially fatal, although its overall incidence is low.
Clinicians have become increasingly aware of the increased risk of SS when prescribing antidepressants such as serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors; however, they may be less likely to consider tricyclic antidepressants and monoamine oxidase inhibitors as causative agents because of other serious side effects that are not related to SS. Drugs outside the antidepressant class, including opiate analgesics such as Demerol (meperidine) and migraine drugs such as triptans, also pose a risk of SS. SS rarely occurs with use of a single agent; it is more commonly the result of a drug-drug interaction. Patients must be educated about the risk of self-medicating with OTC drugs, such as those that contain dextromethorphan, and herbal products, such as St. John’s wort.3
Insomnia
Insomnia is a common sleep disorder that is more prevalent in women and elderly people. Prevalence estimates vary based on specific definitions, assessment procedures, and patients studied; however, population-based studies indicate that about 30% of adults have experienced some degree of insomnia. Insomnia is a common complaint in primary care offices, with up to 40% of patients who seek intervention reporting significant sleep disturbances. Insomnia can be caused by medical conditions, but it is often a neurologic side effect of drugs.9
Patients with insomnia may report trouble falling asleep, difficulty staying asleep, or waking up too early. No matter which sleep phase is impacted, all patients with insomnia report poor quality of sleep overall. Normal sleep architecture consists of non–rapid eye movement (REM) sleep and REM sleep, which cycle throughout the night. Insomnia, a disruption of this sleep architecture, reduces a patient’s quality of life, increases the risk of comorbid medical and psychiatric illness, and poses an increased risk of accidental injury and/or death as a result of reduced ability to perform cognitive tasks. Stimulants are well known to cause insomnia; however, other drugs, including antidepressants, corticosteroids, beta agonists, and antiparkinsonian agents, also should be evaluated when a treatment plan for insomnia is being developed.3,10
Once the provider identifies a potential drug contribution, it is prudent to remove the offending agent, if possible. When discontinuation is not indicated, lowering the dosage, administering the dose early in the day, and educating the patient about sleep-hygiene techniques should be implemented, along with nonpharmacologic interventions to help the patient achieve resolution. Clinical interventions that require sleep-inducing agents are often needed. Drugs that are used to decrease sleep latency (e.g., diphenhydramine and benzodiazepines) can also alter other phases of the sleep cycle, resulting in continued neurologic side effects of excessive daytime sleepiness and next-day drowsiness. Some of the newer agents (Z-drugs, or Z-hypnotics), such as Lunesta (eszopiclone), appear to have little effect on total sleep architecture and are recommended for short-term use for insomnia.3,10
Conclusion
Prescribers and pharmacists must be vigilant about predicting and identifying neurologic side effects of drugs. Adverse drug effects that are overlooked or misdiagnosed pose significant risk to the patient. These neurologic side effects, if not properly addressed, can result in permanent and/or irreversible conditions; therefore, prompt identification and removal of the offending agent is critical.
REFERENCES
1. Tisdale JE, Miller DA. Drug-Induced Diseases: Prevention, Detection, and Management. Bethesda, MD: American Society of Health-System Pharmacists; 2005:111-201.
2. Grosset KA, Grosset DG. Prescribed drugs and neurological complications. J Neurol Neurosurg Psychiatry. 2004;75(suppl 3):iii2-iii8.
3. Clinical Pharmacology [database]. www.clinicalpharmacology.com. Accessed November 30, 2013.
4. FDA Drug Safety Communication: FDA approves label changes for
antimalarial drug mefloquine hydrochloride due to risk of serious
psychiatric and nerve side effects.
www.fda.gov/Drugs/DrugSafety/ucm362227.htm. Accessed September 29, 2013.
5. American Geriatrics Society 2012 Beers Criteria Update Expert
Panel. American Geriatrics Society updated Beers Criteria for
potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
6. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24:155-162.
7. Epilepsy Foundation. About epilepsy. www.epilepsyfoundation.org/aboutepilepsy/index.cfm. Accessed September 29, 2013.
8. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25:91-110.
9. Morin CM, Jarrin DC. Epidemiology of insomnia: prevalence, course, risk factors, and public health burden. Sleep Med Clin. 2013;8:281-297.
10. Barkoukis TJ, Avidan AY, eds. Review of Sleep Medicine. 2nd ed. Philadelphia, PA: Butterworth Heinemann Elsevier; 2007:169-184.
To comment on this article, contact rdavidson@uspharmacist.com.





