US Pharm. 2019:44(12):18-24.
ABSTRACT: Drug-induced nutrient depletions are critical to evaluate when pharmacists review the safety and efficacy of patients’ medication therapies. While certain drug-induced nutrient depletions are well known by pharmacists, many are underdiscussed and subsequently underdiagnosed and undertreated. This article will provide an overview of common drug-induced nutrient depletions for which there is reasonable evidence to support the nutrient deficiency associated with particular medications. Common deficiencies are highlighted, with suggestions for evaluation of clinical significance and patient-care recommendations.
Occurrences of drug-induced nutrient depletions are complex, resulting in inconsistent prevalence among patients taking the same medications with generally the same exposure.1 Nutrient depletions can occur from many pharmacologic treatments, and patients who take more medications may be more likely to have reduced levels of certain nutrients.1,2 While some nutrient depletions may be intentional (i.e., cancer treatments depleting folate), others may result in new comorbidities or unintended consequences.1,3,4 Often the mechanisms by which these depletions occur, and their subsequent outcomes, are not well understood.3 Although these nutrients are present in common foods, they are not present in sufficient levels or are present with bioavailability challenges.5 Therefore patients may require supplementation to avoid deficiencies.
Pharmacists are expected to evaluate the appropriateness of patients’ medications in terms of correct dosage, safety, drug interactions, and other potential benefits and risks associated with medication use; however, evaluation of the implications of drug-induced nutrient depletion is not as commonly performed, and less information is available to support pharmacists’ attempts to do so.6 Drug utilization review (DUR) is a central role of the pharmacist, particularly in the community pharmacy setting.7 DUR is defined as the review of the appropriateness of a medication prior to being dispensed to a patient. The DUR process should screen for drug-drug interactions, therapeutic duplication, drug-disease interactions, drug-allergy contraindications, clinical abuse and misuse, inappropriate dose and duration, and drug-food interactions.7,8 Patients and clinicians are likely unaware of potential nutrient depletions associated with medication use, providing an additional opportunity for pharmacists to contribute to interdisciplinary care of patients.9
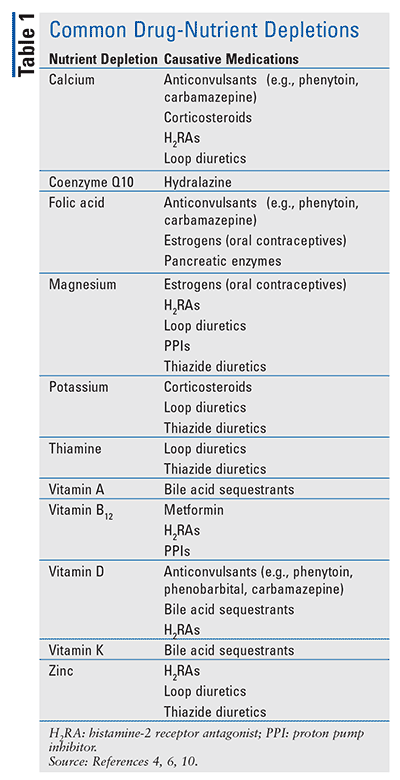
TABLE 1 provides a summary of major drug-induced nutrient depletions together with potentially causative medications. Specific nutrient depletions have been associated with certain medication exposures; however, overall medication burden has also been found to be a risk factor for nutrient depletions. Elderly patients who take 3 or more medications have been found to be more likely to have vitamin D, B1 (riboflavin), B2 (thiamin), B6 (pyridoxine), B9 (folic acid) and B12 (cobalamin) deficiencies.3 By using personalized, predictive, and preventive medicine, nutrient depletions can be identified, managed, and prevented in populations at risk through long-term fortification programs.4 TABLE 2 provides suggested dosing ranges for common nutrient depletions that are not subject to variation in recommended dosages based on associated cause of deficiency or the indication the supplementation is trying to prevent.
NUTRIENT DEPLETIONS
Calcium
Long-term use of corticosteroids can decrease calcium absorption and increase renal excretion of calcium. This may impair bone formation and increase fracture risk. In patients who take corticosteroids long-term for chronic conditions (e.g., rheumatoid arthritis), supplementation is generally required. Deficiency should be verified before supplementation is offered, and vitamin D supplementation should be considered as well to improve calcium absorption.10 Benzodiazepines can also cause decreased calcium absorption and increase the metabolism of vitamin D.2
Many anticonvulsant medications have been associated with hypocalcemia, supported by radiographic, histologic, and biochemical evidence.11,12 Those most frequently associated with hypocalcemia and decreased bone mineral density are medications that are inducers of the cytochrome P450 enzyme (phenobarbital, carbamazepine, phenytoin). Valproate has also been associated with hypocalcemia. No single mechanism of action explains this hypocalcemia and the subsequent bone-health implications. All patients with long-term antiepileptic use should be monitored for bone disease.11
Coenzyme Q10 (CoQ10)
CoQ10 is an essential enzyme that is necessary for energy production. CoQ10 also has antioxidant properties and is a foundational gene regulator in muscle tissue. Clinical manifestations of deficiency can include various cardiovascular complications (hypertension, cardiomyopathy), fatigue, weakness, decreased immune function, and loss of cognitive function.1 Beta blockers have been found to decrease production of this enzyme.13 Evidence regarding statin-induced CoQ10 depletion is inconclusive. Some studies have shown decreases of up to 54% in CoQ10, and this is thought to be a dose-related effect.1 Furthermore, decreased CoQ10 levels in the blood have been shown to be associated with statin-induced myalgia. However, CoQ10 supplementation does not always improve statin-induced muscle pain.10 Other medications with potential impact on CoQ10 levels are metformin, sulfonylureas, and thiazide diuretics.1
Folic Acid (B9)
Folic acid, or folate, is also commonly referred to as B9. It is a water-soluble vitamin. Folate deficiency can pre-sent as megaloblastic-macrocytic anemia.4 Several medications can cause folate deficiency. Most pharmacists are familiar with folate deficiency associated with methotrexate, which inhibits dihyrofolic acid reductase; however, management strategies are unclear.1,14 Low-dose methotrexate has been well tolerated for long periods of time without significant side effects, although many healthcare providers may recommend OTC folic acid supplementation to mitigate side effects of this antifolate therapy.15 In practice though, supplementation often requires higher methotrexate doses for clinical benefit. Toxic effects of methotrexate do occur, though, when folic acid deficiency exists.15 Pharmacists should ensure folic acid levels are frequently monitored in patients taking methotrexate. Sulfamethoxazole has also been found to contribute to folate deficiency.16 The clinical significance of this deficiency may not be frequently relevant, owing to the limited duration of therapy for antibiotics.
Another commonly used medication that can result in folate deficiency is estrogen. It reduces absorption of folic acid and increases excretion as well. In women who have adequate intake of folic acid, this is likely clinically insignificant.1 However, since the CDC recommends that all women of child-bearing potential receive folic acid supplementation, pharmacists should ensure women who are taking oral contraceptives are also taking folic acid supplements.17 Estrogen can also cause several other deficiencies, including deficiencies in vitamins B2 (riboflavin), B6 (pyridoxine), B12 (cobalamin), vitamin C, vitamin E, magnesium, selenium, and zinc. These deficiencies can contribute to side effects experienced with oral contraceptives.5 Pancreatic enzymes can reduce absorption of folic acid and are another common medication that causes deficiency. Pharmacists should monitor for depletion.1 Inversely, folic acid can reduce levels of anticonvulsants, increasing the risk of breakthrough seizures.1 However, this is not a class-wide effect, as some anticonvulsants (carbamazepine, phenytoin) reduce absorption of folic acid while increasing excretion, which puts patients at risk for folic acid deficiency. Some patients may require supplementation.10 Pharmacists should use drug-information resources for each anticonvulsant to understand its interaction with folic acid and subsequent management.
Magnesium
Decreased magnesium absorption has been found in patients who take proton-pump inhibitors (PPIs), especially when duration of use exceeds 1 year. While levels should be checked before recommending a magnesium supplement, many patients will require supplementation to stay within normal ranges.10 Magnesium is an important nutrient because it is involved in over 300 enzymatic reactions—including nerve transmission, energy production, temperature regulation, muscle activation, and development of healthy bones and teeth.1 Magnesium also has key physiologic benefits such as blood-pressure regulation, bone development, and muscular activity. Deficiency has even been associated with increased cardiovascular risk, such as hypertension, stroke, heart attack, and atherosclerosis.1
Long-term magnesium deficiency can result in cardiac arrhythmias.18 Although loop diuretics are typically associated with other nutrient deficiencies (calcium, potassium), magnesium does typically drop 4.7% to 11% with loop-diuretic therapy, although clinical trials have generally not found this to be clinically significant.19 Thiazide diuretics can be another source of depleted magnesium levels. While literature is controversial regarding clinical significance, enough studies have shown magnesium-depleting effects to screen for depletion in patients taking thiazide diuretics.1,20
Potassium
Corticosteroids can cause potassium depletion in addition to calcium depletion. Some patients may require potassium-chloride supplementation, but deficiency should be verified before supplementation is started, due to risks associated with hyperkalemia.10 As pharmacists are aware, many diuretics (loop diuretics, thiazide diuretics) can cause potassium depletion. Potassium levels should be monitored, and potassium supplementation is frequently required in patients taking loop diuretics.10
Vitamin B6 (Pyridoxine)
Vitamin B6 is an essential cofactor in many enzymatic reactions. Deficiency is rare in healthy individuals. Symptoms of deficiency include weakness, dizziness, irritability, inflammation, bilateral limb numbness, and development of confusion. 21 Estrogens (oral contraceptives, hormone replacement therapy) interfere with metabolism of vitamin B6; however, low-dose oral contraceptives seem to naturally correct for this deficiency.6
Vitamin B12 (Cobalamin)
Vitamin B12 is an essential nutrient for cardiovascular, neurologic, and hemopoietic function.22 Vitamin B12 requires intrinsic factor (IF) for transportation to the small intestine and absorption into the blood stream. IF is produced in the gut.6 Bile acid sequestrants can bind IF-vitamin B12 complexes; however, absorption of vitamin B12 is not completely impaired, and this is unlikely to be clinically significant.6 Deficiency can result from a lack of IF or hydrochloric acid in the stomach that is required to liberate vitamin B12 from its bound state.23 Vitamin B12 deficiency has been associated with multiple metabolic abnormalities, such as insulin resistance and defective neurotransmitter synthesis.24 Deficiency is prevalent among patients with both type 1 and type 2 diabetes.22 This is particularly important to monitor for, considering that metformin use has been linked to vitamin B12 deficiency and prevalence ranges from 5.8% to 33%, although only 2.6% of patients supplement vitamin B12.22 Vitamin B12 levels have been shown to decrease by at least 22% and are not seen with other diabetes therapies, such as sulfonylureas.22
The risk of development of metformin-induced vitamin B12 deficiency increases with age, vegetarian diet, metformin dose, and duration of use (>3 years).3,6,22 Vitamin B12 levels can start to decrease as early as 3 months into metformin therapy; however, clinical significance typically does not become evident until 5 to 10 years of therapy due to large stores in the liver that are slowly depleted.22 Deficiency has also been associated with an increased risk of gestational diabetes and peripheral neuropathy.24,25 Guidelines currently do not recommend or advocate for routine screening among patients with type 2 diabetes; however, screening could be considered at baseline and in patients who are elderly, who are taking high doses (2 g/day), or who have long-term metformin use (>3-4 years) or worsening neuropathy.22
Like metformin, histamine-2 receptor antagonists (H2RAs) can result in vitamin B12 deficiency, because they can decrease absorption of vitamin B12. While this is clinically insignificant with short-term or occasional use, patients who use H2RAs long-term (>2 years) should be assessed for depletion. Supplementation may be required, particularly in patients taking high doses for prolonged time periods. PPIs can also cause vitamin B12 deficiency, but as with H2RAs, it is likely clinically insignificant.23 Gastric acid is required to cleave vitamin B12 from dietary proteins through pepsin. It has been hypothesized that since pepsin is activated from its precursor by an acidic pH, acid could lead to vitamin B12 deficiency from achlorhydria and atrophic gastritis.26
Colchicine is another medication that can cause decreased vitamin B12 through disruption that results in malabsorption.6 This is not likely to occur in clinical practice because doses of 1.9 to 3.9 mg/day are required for deficiency to develop; these are higher than commonly used doses. Vitamin B12 deficiency is important to prevent, identify, and treat, because both vitamin B12 and folate can elevate homocysteine levels.1 Supplementation of these nutrients has been found to reduce homocysteine levels and subsequently ameliorate insulin resistance.27
Vitamin D
Vitamin D is inactivated by many anticonvulsants, such as carbamazepine. This leads to decreased vitamin D levels through dihydroxy–folic acid reductase inhibition.11,12 In turn, this can slow the rate of calcium absorption from the gut, putting patients at risk for both calcium and vitamin D deficiency. Supplementation should be considered in patients who take anticonvulsants more than 6 months after deficiency is verified.10
Vitamin K
Bile acid sequestrants reduce absorption of all fat-soluble vitamins (vitamins A, D, E, K) from the gut. Vitamin A deficiency will most commonly present with visual disturbances.4
Depletion should be monitored in patients who take warfarin, as warfarin’s anticoagulant effects are subsequent to its vitamin K antagonist activity.10,28 This is especially concerning in patients who may have international normalized ratios (INRs) that trend higher because the deficiency of vitamin K activity will continue to elevate the INR. The consistency of dietary intake of vitamin K should also be frequently reviewed with patients taking warfarin.28
Orlistat has also been evaluated for its impact on fat-soluble vitamins. Orlistat works to produce weight-loss effects through decreasing the absorption of fat-containing foods in the diet.29 As a result, absorption of fat-soluble vitamins could result. McDuffie and colleagues evaluated doses of orlistat 120 mg administered three times a day in adolescents. Patients were also provided supplementation of vitamin A 5,000 IU daily; vitamin D 400 IU daily; vitamin E 300 IU daily; and vitamin K 25 mcg. Vitamin E absorption was found to be impaired, but serum levels were not found to be significantly decreased from baseline. Vitamin K levels decreased but were not significant. Significant reductions in vitamin D were found compared with baseline after 1 month of therapy.30
Zinc
Zinc deficiency has been associated with angiotensin converting enzyme (ACE) inhibitor use when these medications are used for 6 months or longer.3 Deficiency can also occur with diuretic use that is 4 weeks or longer in duration.3 Zinc deficiency is important; emerging evidence suggests that it may play a role in the development of metabolic syndrome.25 Zinc is involved in over 300 enzymatic reactions and plays a critical role in cell gene expression specific to immune cells.1,31 Zinc deficiency has been found to increase production of tumor necrosis factor alpha, interleukin (IL) beta and IL-8. Increased production of these inflammatory cytokines produces increased production of metabolic markers.2,31 Medications that may warrant zinc monitoring include ACE inhibitors, loop diuretics, and thiazide diuretics.1
Addressing Nutrient Deficiencies
Widespread supplementation of nutrients is not recommended without establishing nutrient deficiencies or identifying that the patient is at high risk for a nutrient deficiency. When evaluating potential drug-induced nutrient deficiencies, the first step pharmacists should take is to assess whether the deficiency is subclinical or clinically relevant.32 Are there potential health implications if this deficiency is not corrected, or is the deficiency minor and unlikely to result in health consequences? Next, evaluate whether therapy is still appropriate or if an alternative medication could be used for symptom/disease state control without these risks.26 For example, does the patient truly need a PPI or would discontinuation be warranted? Additionally, pharmacists should assess patient diets and address potential dietary intake issues that could be contributing to nutrient deficiencies.33
Conclusion
Drug-induced nutrient depletions are common occurrences in patient medication use and should be monitored for and appropriately managed when deficiencies are identified. Although nutrient depletions can have clinically significant implications for patients, supplementation should not occur until a deficiency has been verified (i.e., via laboratory testing), reasonable evidence exists to suspect deficiency, or there is limited risk with supplementation when a deficiency has not been verified.
REFERENCES
1. LaValle JB. Hidden disruptions in metabolic syndrome: drug-induced nutrient depletion as a pathway to accelerated pathophysiology of metabolic syndrome. Altern Ther Health Med. 2006;12(2):26-31.
2. Pharmavite. Common drug classes, drug-nutrient depletions, & drug-nutrient interactions. www.aafp.org/dam/AAFP/documents/about_us/sponsored_resources/Nature%20Made%20Handout.pdf. Accessed September 20, 2019.
3. Reilly W, Ilich J. Prescription drugs and nutrient depletion: how much is known? Adv Nutr. 2017;8:23.
4. Karadima V, Kraniotou C, Bellos G, Tsangaris GT. Drug-micronutrient interactions: Food for thought and thought for action. EPMA J. 2016;7:10.
5. Palmery M, Saraceno A, Vaiarelli A, Carlomagno G. Oral contraceptives and changes in nutritional requirements. Eur Rev Med Pharmacol Sci. 2013;17:1804-1813.
6. Smiley T. Drug-induced nutrient depletion. https://tevapharmacysolutions.com/sites/default/files/pdf/ratio_nutrient_20090226.pdf. Accessed September 20, 2019.
7. Castillo S, Begley K, Hoie E, et al. “Brown bag” simulations to teach drug utilization review. Am J Pharm Educ. 2014;78:40.
8. Thompson JE. A Practical Guide to Contemporary Pharmacy Practice, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
9. Cass H. A practical guide to avoiding drug-induced nutrient depletion. Nutrition Review. Updated December 11, 2016. https://nutritionreview.org/2016/12/practical-guide-avoiding-drug-induced-nutrient-depletion/. Accessed September 20, 2019.
10. Natural Medicines. Drug-induced nutrient depletions. https://naturalmedicines.therapeuticresearch.com/tools/charts/drug-induced-nutrient-depletions.aspx. Accessed September 20, 2019.
11. Pack AM. The association between antiepileptic drugs and bone disease. Epilepsy Curr. 2003;3(3):91-95.
12. Van Zyl M. The effects of drugs on nutrition. S Afr J Clin Nutr. 2011;24(3):S38-S41.
13. Kishi T, Watanabe T, Folkers K. Bioenergetics in clinical medicine XV: inhibition of coenzyme Q10-enyzmes by clinically used adrenergic blockers of beta-receptors. Res Commun Chem Pathol Pharmacol. 1977;17:157-164.
14. Methotrexate package insert. Huntsville, AL: DAVA Pharmaceuticals; 2016.
15. Manna R, Verrecchia E, Diaco M, et al. Folic acid supplementation during methotrexate treatment: nonsense? Rheumatology (Oxford). 2005;44:563-564.
16. Ho JMW, Juurlink DN. Considerations when prescribing trimethoprim-sulfamethoxazole. CMAJ. 2011;183(16):1851-1858.
17. CDC. Folic acid recommendations. Updated August 13, 2019. www.cdc.gov/ncbddd/folicacid/recommendations.html. Accessed September 22, 2019.
18. Leary WP, Reyes AJ. Diuretic-induced magnesium loss. Drugs. 1984;28(Suppl 1):182-187.
19. Davies DL, Fraser R. Do diuretics cause magnesium deficiency? Br J Clin Pharmacol. 1993;36:1-10.
20. Atsmon J, Dolev E. Drug-induced hypomagnesaemia. Drug Safety. 2005;28:763-788.
21. Medscape. Pyridoxine deficiency. www.emedicine.com/med/TOPIC1977.htm. Accessed September 20, 2019.
22. Kibirige D, Mwebaze R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? J Diabetes Metab Disord. 2013;12:17.
23. National Institutes of Health. Office of Dietary Supplements. Vitamin B12. Ods.od.nih.gov/factsheets/VitaminB12_pf.asp. Accessed September 20, 2019.
24. Kouroglou E, Anagnostis P, Daponte A, Bargiota A. Vitamin B12 insufficiency is associated with increased risk of gestational diabetes mellitus: a systematic review and meta-analysis. Endocrine. 2019; in press.
25. Alvarez M, Rincon O, Saavedra G, Moreno SM. Vitamin B12 deficienecy and diabetic neuropathy in patients taking metformin: across-sectional study. Endocrin Connect. 2019; in 8(10):1324-1329.
26. Heidelbaugh JJ. Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. Ther Adv Drug Saf. 2013;4(3):125-133.
27. Setola E, Monti LD, Galluccio E, et al. Insulin resistance and endothelial function are improved after folate and vitamin B12 therapy in patients with metabolic syndrome: relationship between homocysteine levels and hyperinsulinemia. Eur J Endocrinol. 2004;151:483-489.
28. Coumadin (warfarin sodium) package insert. Princeton, NJ: Bristol-Myers Squibb Company; 2017.
29. Alli (orlistat) package insert. Moon Township, PA: Glaxo-SmithKline; 2007.
30. McDuffie JR, Calis KA, Booth SL, et al. Effects of orlistat on fat-soluble vitamins in obese adolescents. Pharmacotherapy. 2002;22(7):914-922.
31. Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45-62.
32. Mohn ES, Kern HJ, Saltzman E, et al. Evidence of drug-nutrient interactions with chronic use of commonly prescribed medications: an update. Pharmaceutics. 2018;10:36.
33. Krinsky DL, LaValle JB, Hawkins E, et al. Natural Therapeutic Pocket Guide, 2nd ed. Hudson, Ohio: Lexi-Comp, Inc; 2003.
To comment on this article, contact rdavidson@uspharmacist.







