US Pharm. 2018;9(43)8-12.
Unintended pregnancy, that is, a pregnancy that is either mistimed or unwanted, is a common occurrence in the United States. Approximately 45% of all pregnancies are considered to be unintended, with 2.8 million unintended pregnancies in 2011 alone.1 Most unintended pregnancies can be attributed to nonuse or misuse of contraception.2 Emergency contraception (EC), also referred to as the morning-after pill, has been used to prevent such pregnancies since the 1960s.3
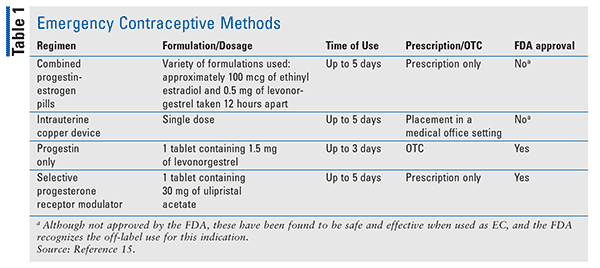
EC is any form of contraception, hormonal or nonhormonal, that can be used to prevent a pregnancy.4 Currently, there are four methods of EC approved or recognized as safe and effective by the FDA (Table 1): the Yuzpe method (combined oral contraceptive pills); progestin-only pills containing levonorgestrel; ulipristal acetate; and a copper intrauterine device (IUD).
Use of EC is slowly increasing. From 2011 to 2015, 20% of women of childbearing age reported having ever used EC, compared with 11% of women from 2006 to 2010.5 In 2002 only 4% of women reported having used EC, and in 1995 this number was less than 1%.5 This growth can most likely be attributed to EC’s increased availability over the years; in 2006 levonorgestrel was approved for OTC use in women aged 18 years and older.6 More recently, in 2013, the FDA approved single-dose levonorgestrel products to be available OTC with no restrictions.7 In addition, there are some states that allow pharmacists to prescribe and provide EC directly to women. With this increased availability of EC products, pharmacists play an important role in educating patients about EC and its safe and effective use.
REGIMENS
Combined Progestin-Estrogen Pills
The Yuzpe regimen, first described in 1974, uses a combination of approximately 100 mcg of ethinyl estradiol and 0.5 mg of levonorgestrel every 12 hours for two doses.8 This method is most effective when used within 72 hours but can be considered for use up to 120 hours after unprotected intercourse.9,10 The primary mechanism of action is thought to be through the inhibition or delay of ovulation.11 Emergency contraceptive effectiveness using this regimen ranges from 44.2% to 88.7%, depending on the timeliness of use and day of the menstrual cycle.12 In 1998, the FDA approved the first EC-dedicated product, which mimicked the Yuzpe regimen, containing both estrogen and progestin tablets administered in two doses, but this product was withdrawn from the market in 2004.13 Currently, there are a number of birth-control pills that can be used for emergency contraception. Research has also demonstrated that other progestins, such as norethindrone, in combination with ethinyl estradiol, may be used as EC when levonorgestrel is not available.14
Progestin-Only Pills
In 1999, the FDA approved the first EC product to contain only progestin, levonorgestrel pills.13 In 2013 this became readily available OTC, making Plan B One-Step and its generic equivalents the most commonly available EC.15 Levonorgestrel is administered as a one-time dose of 1.5 mg within 72 hours of unprotected intercourse. Data from clinical trials demonstrate this regimen to be more effective when compared with the Yupze regimen.16,17 Similarly, the mechanism of action is through the delay or inhibition of ovulation; once the luteinizing-hormone surge has occurred, levonorgestrel has no effect on ovulation.17
Ulipristal
Ulipristal acetate (ella) has been available since December 2010. It is a progesterone receptor modulator that delays or prevents ovulation. This agent may also have a direct inhibitory effect on follicular rupture, allowing it to be effective even when administered just before ovulation, unlike levonorgestrel. Its different mechanism of action allows it to maintain its effectiveness with time, working as well on day 4 after unprotected intercourse as it does on day 1.18,19 Ulipristal is available as a 30-mg tablet and requires a prescription. It is administered as a single dose within 120 hours of unprotected intercourse.
Copper IUDs
The copper IUD is another option available for EC, although it is not approved by the FDA for this indication. The copper IUD (ParaGard) is considered the most effective option for EC. A review of 42 studies demonstrated the pregnancy rate to be less than 0.1% after insertion.20 The IUD should be placed within 5 days; some studies have indicated effectiveness up to 10 days after intercourse.15 The IUD has the additional benefit of providing continued contraception for up to 10 years after placement.21 The primary mechanism of action of the copper IUD is the prevention of fertilization; a cytotoxic inflammatory reaction decreases sperm motility and viability, reducing the sperm’s ability to fertilize an ovum.22,23
DRUG INTERACTIONS
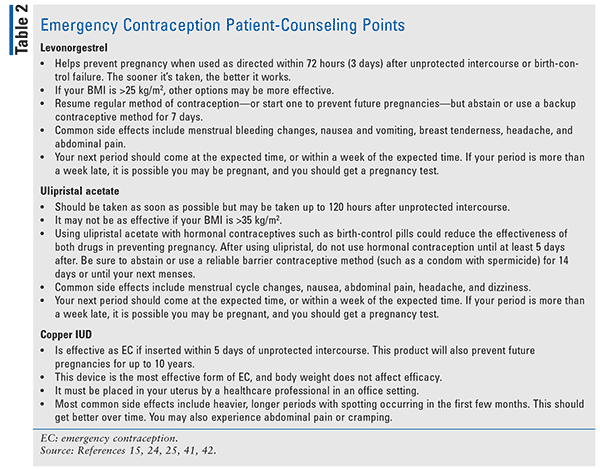
Strong inducers of CYP3A4 may reduce the effectiveness of ECs containing ulipristal, progestin only, or a combination of ethinyl estradiol and a progestin. Women who are taking medications such as rifampin, anticonvulsants, antifungals, antiretrovirals or who are taking the supplement St. John’s wort should be advised of the potential for reduced efficacy of EC.24
Ulipristal is a progesterone receptor modulator, blocking progestin. Because of this, there are implications for starting progestin-containing hormonal contraceptives immediately after ulipristal use. Patients should wait at least 5 days after taking ulipristal, and a reliable barrier-contraception method should be used until the next menstrual period.25
ADVERSE EFFECTS
There have been no deaths or serious complications causally linked to EC use.15 Nausea and vomiting are the most common adverse effects associated with EC use, with the combined
estrogen-progestin regimen having a significantly higher rate compared with levonorgestrel and
ulipristal regimens.17
Another common side effect is menstrual cycle changes following the use of EC; these changes may vary depending on which point in the menstrual cycle the pills are taken.26-28 When levonorgestrel was taken early in the cycle, it shortened cycle length. When taken later in the cycle, it either had no effect or appeared to lengthen the cycle. Women who had used ulipristal menstruated 2 days later than expected compared with levonorgestrel users who experienced menstruation 1 day earlier, on average.29
Women using the copper IUD may also experience bleeding irregularities; however, evidence suggests that this may decrease over time.30 Patients may also experience pain upon insertion as well as cramping afterwards.
SAFETY AND CONTRAINDICATIONS
There are no reported cases of birth defects caused by EC use. Studies evaluating the use of oral contraceptives and potential fetal harm have found no associations.31,32 According to the CDC’s publication, U.S. Medical Eligibility Criteria for Contraceptive Use, 2016, there are no conditions where the use of EC is strictly prohibited, except for known pregnancy.24 Because of the very short duration of exposure and overall low hormonal content of a combined EC regimen, treatment can be considered safe for those women who would ordinarily be cautioned against taking oral contraceptives, such as those who have migraines or cardiovascular disease, or who are breastfeeding. Breastfeeding, however, should be avoided for 24 hours after taking ulipristal.
Contraindications to the use of the copper IUD are the same, regardless of the reason for use.
BODY WEIGHT CONSIDERATIONS
There are questions regarding the efficacy of EC in patients with a higher BMI. In 2011, an analysis demonstrated decreased efficacy for women with higher BMIs for both levonorgestrel and ulipristal; women using levonorgestrel with a BMI of >30 kg/m2 had a failure rate of 5.6%; those who used ulipristal had a failure of 2.6%.33 The authors suggested that levonorgestrel may be ineffective for women with a BMI of >26 kg/m2, while ulipristal may be less effective in those with a BMI >30 kg/m2, although some data suggest that observed pregnancy rates appear to be no different than expected at a BMI up to 35 kg/m2.33,34 Another study reported similar results regarding levonorgestrel EC use; women weighing >80 kg had a pregnancy risk of 6%, equivalent to the risk of pregnancy if no contraception was used.35 A recent World Health Organization publication reported data finding a higher failure rate in obese women who used 1.5 mg of levonorgestrel compared with women with normal BMI levels.36 Additionally, pharmacokinetic studies have found that serum concentrations of levonorgestrel were decreased by 50% in obese women compared with women who have a normal BMI; however, blood levels for ulipristal were similar among both groups.37,38
Despite these findings, the FDA currently concludes that the available evidence is too limited and not robust enough to make a final determination regarding use of EC regimens containing levonorgestrel and ulipristal in obese women. The benefits of taking these medications still outweigh any risks.39
Patients who are overweight or obese should be counseled on the potential decrease in efficacy of levonorgestrel- and ulipristal-containing EC products, especially as BMIs increase over 30 kg/m2 or in patients who weigh >75 kg. In these patients, a copper IUD should be considered because its efficacy is not influenced by weight. If the IUD is not an option owing to accessibility or contraindications, ulipristal may be more efficacious than levonorgestrel.
ROLE OF THE PHARMACIST
Despite the increased use and availability of EC, the number of women receiving education from healthcare providers has not changed; approximately 3% of women report ever having been counseled.40 Pharmacists have the opportunity to fill this gap. With increased accessibility, the pharmacist may be the only healthcare provider that EC users encounter. Almost two-thirds of EC users indicated that they had purchased it without a prescription.40 Pharmacists, especially in the community, can help educate patients on the available methods of EC and their effectiveness and proper use, and address any concerns and barriers to EC use.
During counseling, pharmacists should indicate that EC products do not prevent against sexually transmitted diseases and that these agents are not meant for deliberate, repeated use or routine use as a contraceptive method. The pharmacist should take this opportunity to provide education about other methods of contraception; for example, at this time, women may be educated on the copper IUD. Patients should also be advised that these products are not abortifacients. They are meant to prevent a pregnancy from happening; they will not harm an already existing pregnancy. See Table 2 for additional counseling points.
The use of EC will continue to increase. Pharmacists play an important role in providing patient education about EC because many women will likely go to the pharmacy first. Pharmacists need to feel comfortable speaking with their patients about this sensitive issue. As frontline healthcare providers, pharmacists will continue to fill an important gap in this area, having the ability to reach out to women seeking information and education regarding emergency contraception.
What are the different types of emergency contraception?
There are two main forms of emergency contraception: pills and the copper intrauterine device (IUD). The copper IUD should be placed into your uterus by a doctor up to 5 days after having unprotected intercourse and can be left there for up to 10 years to prevent pregnancy.
There are three different types of pills that can be used. Regular birth-control pills can be taken at higher doses. You will need to discuss with your doctor which birth-control pills and how many to take. They are given in two doses, 12 hours apart. They should be taken within 72 hours after having unprotected intercourse.
Pills containing the hormone levonorgestrel are sold OTC. You do not need a prescription to purchase them. One common brand is PlanB One-Step. There are other brands and many generic versions available as well. This is a single-dose pill and should also be taken within 72 hours of unprotected intercourse.
Ulipristal (brand name: ella) is available only by prescription. It is a single dose but can be taken up to 5 days after having unprotected intercourse.
How do they work?
The pills work by stopping your ovaries from releasing an egg. The IUD works by stopping the sperm from fertilizing the egg. If you are already pregnant, emergency contraception will not end the pregnancy or hurt the growing baby.
What are the side effects?
Nausea and vomiting are the most common side effects, especially if you are using regular birth-control pills. Levonorgestrel pills produce less nausea and vomiting. You may also have irregular bleeding, spotting, breast tenderness, and headache.
The copper IUD may cause heavier and longer periods, but this should get better after a few months.
What happens after I take emergency contraception?
Your next period should come at the expected time, or within a week of your expected time. If your period is more than a week late, it is possible you may be pregnant, and you should get a pregnancy test.
REFERENCES
1. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843-852.
2. Guttmacher Institute. Unintended pregnancy in the United States. Published January 26, 2012. www.guttmacher.org/fact-sheet/unintended-pregnancy-united-states. Accessed July 30, 2018.
3. Van Look PF, von Hertzen H. Emergency contraception. Br Med Bull. 1993;49(1):158-170.
4. Emergency contraception. World Health Organization. www.who.int/news-room/fact-sheets/detail/emergency-contraception. Accessed August 3, 2018.
5. Daniels K, Jones J, Abma J. Use of emergency contraception among women aged 15-44: United States, 2006-2010. NCHS Data Brief. 2013;(112):1-8.
6. FDA. Press announcements. FDA approves over-the-counter access for Plan B for women 18 and older. https://wayback.archive-it.org/7993/20170112033126/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm108717.htm. Accessed August 3, 2018.
7. FDA. Press announcements. FDA approves Plan B One-Step emergency contraceptive for use without a prescription for all women of child-bearing potential. https://wayback.archive-it.org/7993/20170112033121/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm358082.htm. Accessed August 3, 2018.
8. Yuzpe AA, Thurlow HJ, Ramzy I, Leyshon JI. Post coital contraception—a pilot study. J Reprod Med. 1974;13(2):53-58.
9. Rodrigues I, Grou F, Joly J. Effectiveness of emergency contraceptive pills between 72 and 120 hours after unprotected sexual intercourse. Am J Obstet Gynecol. 2001;184(4):531-537.
10. Ellertson C, Evans M, Ferden S, et al. Extending the time limit for starting the Yuzpe regimen of emergency contraception to 120 hours. Obstet Gynecol. 2003;101(6):1168-1171.
11. Trussell J, Ellertson C, Dorflinger L. Effectiveness of the Yuzpe regimen of emergency contraception by cycle day of intercourse: implications for mechanism of action. Contraception. 2003;67(3):167-171.
12. Trussell J, Raymond EG. Statistical evidence about the mechanism of action of the Yuzpe regimen of emergency contraception. Obstet Gynecol. 1999;93(5 pt 2):872-876.
13. FDA. Bioterrorism and drug preparedness. FDA’s decision regarding Plan B: questions and answers. www.fda.gov/drugs/emergencypreparedness/bioterrorismanddrugpreparedness/ucm109795.htm. Accessed August 3, 2018.
14. Ellertson C, Webb A, Blanchard K, et al. Modifying the Yuzpe regimen of emergency contraception: a multicenter randomized controlled trial. Obstet Gynecol. 2003;101(6):1160-1167.
15. American College of Obstetricians and Gynecologists. Practice Bulletin No. 152: Emergency contraception. Obstet Gynecol. 2015;126(3):e1-11.
16. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Task Force on Postovulatory Methods of Fertility Regulation. Lancet Lond Engl. 1998;352(9126):428-433.
17. Shen J, Che Y, Showell E, et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2017;8:CD001324.
18. Gemzell-Danielsson K, Berger C, Lalitkumar PG. Emergency contraception—mechanisms of action. Contraception. 2013;87(3):300-308.
19. Kim A, Bridgeman MB. Ulipristal acetate (ella): a selective progesterone receptor modulator for emergency contraception. P T. 2011;36(6):325-331.
20. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod Oxf Engl. 2012;27(7):1994-2000.
21. United Nations Development Programme; United Nations Population Fund; World Health Organization; World Bank, Special Programme of Research, Development and Research Training in Human Reproduction. Long-term reversible contraception: Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341-352.
22. Rivera R, Yacobson I, Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol. 1999;181(5 pt 1):1263-1269.
23. Kaneshiro B, Aeby T. Long-term safety, efficacy, and patient acceptability of the intrauterine Copper T-380A contraceptive device. Int J Womens Health. 2010;2:211-220.
24. Curtis KM, Tepper NK,Jatlaoul TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103.
25. ella (ulipristal acetate) package insert. Paris, France: Laboratoire HRA Pharma; May 2018.
26. Raymond EG, Goldberg A, Trussell J, et al. Bleeding patterns after use of levonorgestrel emergency contraceptive pills. Contraception. 2006;73(4):376-381.
27. Gainer E, Kenfack B, Mboudou E, et al. Menstrual bleeding patterns following levonorgestrel emergency contraception. Contraception. 2006;74(2):118-124.
28. Tirelli A, Cagnacci A, Volpe A. Levonorgestrel administration in emergency contraception: bleeding pattern and pituitary-ovarian function. Contraception. 2008;77(5):328-332.
29. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555-562.
30. Hubacher D, Chen P-L, Park S. Side effects from the copper IUD: do they decrease over time? Contraception. 2009;79(5):356-362.
31. Charlton BM, Mølgaard-Nielsen D, Svanström H, et al. Maternal use of oral contraceptives and risk of birth defects in Denmark: prospective, nationwide cohort study. BMJ. 2016;352:h6712.
32. Waller DK, Gallaway MS, Taylor LG, et al. Use of oral contraceptives in pregnancy and major structural birth defects in offspring. Epidemiology. 2010;21(2):232-239.
33. Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363-367.
34. Moreau C, Trussell J. Results from pooled phase III studies of ulipristal acetate for emergency contraception. Contraception. 2012;86(6):673-680.
35. Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97-104.
36. Festin MP, Peregoudov A, Seuc A, et al. Effect of BMI and body weight on pregnancy rates with LNG as emergency contraception: analysis of four WHO HRP studies. Contraception. 2017;95(1):50-54.
37. Edelman AB, Cherala G, Blue SW, et al. Impact of obesity on the pharmacokinetics of levonorgestrel-based emergency contraception: single and double dosing. Contraception. 2016;94(1):52-57.
38. Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95(5):464-469.
39. FDA. Postmarket drug safety information for patients and providers—Plan B: health care professional questions and answers. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm491172.htm. Accessed August 3, 2018.
40. Kavanaugh ML, Williams SL, Schwarz EB. Emergency contraception use and counseling after changes in United States prescription status. Fertil Steril. 2011;95(8):2578-2581.
41. Teva Women’s Health. Patient counseling guide. June 2017. www.planbonestep.com/Assets/Pdf/PatientCounselingGuide.pdf. Accessed August 3, 2018.
42. ParaGard T380A (intrauterine copper contraceptive) package insert. Trumbull, CT: Cooper Surgical; January 2018.
To comment on this article, contact rdavidson@uspharmacist.com.