US Pharm. 2012;37(5)(Diabetes suppl): 7-10.
In our modern world, diabetes prevalence is on the rise. In 2010, statistics showed that over 25 million people in the United States, including children, have diabetes mellitus.1 Of that population, seven million people who have diabetes are undiagnosed.1 In addition, diabetes prevalence increases with age. Between 2005 and 2008, statistics showed 26.9% of people with type 1 or type 2 diabetes mellitus were over the age of 65 years, while 17.4% comprised those between 20 and 64 years of age.1 At this rate, the number of people diagnosed with diabetes in the world is expected to increase by 114% from the year 2000 to 2030.2 As a result, effective diabetes management will continue to be an important consideration for patients and is key to reducing the risk of complications such as heart disease, blindness, renal disease, and unnecessary amputations.
Proper glycemic control, including self-monitoring of blood glucose (SMBG), is key to managing diabetes. It has been shown that microvascular complications, such as neuropathy, nephropathy, and retinopathy, are reduced 40% for every percentage reduction in hemoglobin A1C values.1 Furthermore, a survey of 1,895 diabetic patients suggested that decreased blood glucose monitoring compliance was observed in patients who had more than two hospitalizations in a 2-year period.3 Yet, despite current evidence of the importance of daily SMBG, many patients who have diabetes do not regularly check their blood glucose at home. For example, up to 67% of patients do not check their blood glucose regularly for reasons such as sore fingers, inconvenience, and the fear of needles.3 Hence, some patients choose to avoid these unpleasant aspects by simply not checking blood sugars on a regular basis, especially since hyperglycemia is often asymptomatic in the early stages of diabetes mellitus.
In addition to the difficulties posed by SMBG, maintaining proper glycemic control can be a challenge, especially for patients who are on insulin therapy. Patients who use short-acting insulin to help control blood glucose during a meal must constantly estimate their insulin doses by counting the carbohydrate content of the meal. Since most of us do not eat the same meal every day for breakfast, lunch, and dinner, counting carbohydrates can become a cumbersome process. Furthermore, improperly estimating an insulin dose can potentially result in undertreatment or overtreatment, which may have grave consequences. Based on discharge data of Californian hospitals, hypoglycemia was found to be responsible for approximately 1.7% of hospitalized diabetic patients.4
Fortunately, with emerging technologies in our modern market, cutting-edge glucose monitoring systems have been developed to aid patients in maintaining SMBG compliance and tight glucose control as well as minimizing potential adverse events. Current technology includes features that aid patients in calculating insulin doses, allow them to connect their blood glucose meters to smartphones, or even check blood glucose levels using noninvasive methods. This article is focused on providing an in-depth look at these technologies by helping pharmacists understand the features of some of the latest blood glucose monitoring systems available and how patients may utilize them to keep their diabetes under control.
FreeStyle InsuLinx
In today’s society, even with better understanding of the importance of glycemic control, only 41% of people with diabetes have the ability to calculate an insulin dose based on carbohydrate intake and blood glucose levels.5 Controlling blood sugar with a fast-acting insulin is difficult because it poses the risk of hypoglycemia or hyperglycemia if insulin is not administered in a correct manner. It is difficult to estimate the amount of insulin required with varying portion sizes and fluctuating sugar levels throughout the day. Therefore, to achieve the proper insulin dose, Abbott Diabetes Care has introduced the FreeStyle InsuLinx Blood Glucose Monitoring System, which has integrated a bolus calculator into the meter (FIGURE 1). It uses insulin pump technology to determine the insulin-to-carbohydrate ratio to help patients accurately estimate their daily insulin requirements.6

Abbott’s new blood glucose monitor is the first technology to calculate the mealtime (rapid-acting) insulin dosage in addition to measuring blood glucose. The device calculates bolus insulin dosages based on target glucose level and carbohydrate intake; however, it is not used for long-acting insulin dosing.7 According to Abbott, “The new FreeStyle InsuLinx System also offers several additional user-friendly features including a touch screen interface, automated logbook, personalization preferences and USB connectivity for plug-and-play reports via the new FreeStyle Auto-Assist data management software.”6 The touch screen requires no hardware keys; hence, the larger screen allows for easier manipulation of the device. Subsequently, it responds to the needs of an individual and allows the patient to set alarms and reminders to manage diabetes in a more efficient manner.7 Furthermore, the logbook functionality assists the patient by recording blood sugar levels, which can be downloaded by connecting the glucose monitor to the computer via USB. In order to achieve this, the system comes with FreeStyle Auto-Assist software compatible with PCs and Macs. This feature provides the opportunity for health care professionals to manage diabetes with reports, reminders, and messages.6 In addition, the FreeStyle InsuLinx meter needs to be programmed by a physician or a diabetes nurse specialist to set up the target glucose goal for the patient to reduce any adverse effects of insulin.7
Diabetes is a difficult disease to manage. Patients have to constantly calculate their insulin requirements based on various meals consumed throughout the day. The carbohydrate content may vary greatly between meals, which may pose a challenge to patients. The FreeStyle InsuLinx meter provides the flexibility of estimating insulin dosages, which allows patients to relax about their meal without having to worry about portion sizes and how these may affect their blood sugar levels. Currently, the FreeStyle InsuLinx is approved in Europe and was also recently approved by the FDA for use in the U.S. Abbott is planning to release the product to U.S. consumers in the coming months.8 Although no efficacy data are available for the device, Abbott claims that it can accurately compute an insulin dose utilizing the bolus calculator feature.8
iBGStar
With new and innovative technologies on the horizon, smartphones are ever increasing in popularity among consumers around the world. A recent study in the last quarter of 2011 has shown that approximately 91% of mobile users own a smartphone, powered either by Android or iOS operating systems.9 Smartphones have become so widespread that they have become a vital tool in people’s lives. In addition to calling capabilities, smartphones can be used to browse the Internet, check e-mail, set appointments, and perform many other functions on a daily basis. Performing daily tasks becomes more convenient with the ability to access myriad functions and store data in one convenient, pocket-sized device. But what about using such a device for helping maintain a medical condition like diabetes? That’s where Sanofi US and AgaMatrix have partnered to offer the iBGStar Glucose Monitoring System (FIGURE 2).
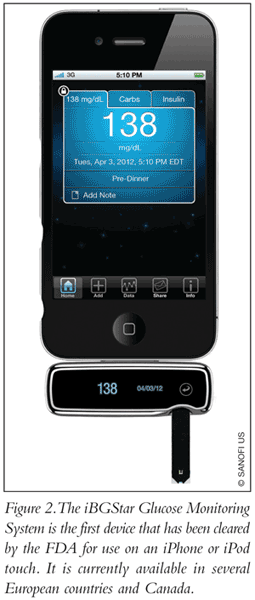
As the “i” in iBGStar suggests, the glucose monitor is specifically made for the Apple iPhone or iPod touch. iBGStar is the first device that has been cleared by the FDA for use on an Apple device, and it is currently available in some European countries.10 The device has also been recently introduced in Canada at a price point of CAD $64.99, and with the recent FDA 510(k) clearance, it is expected to launch in the U.S. soon.11 However, there are currently no data available to indicate costs or insurance coverage related to the device in the U.S.11 The iBGStar uses its Diabetes Manager App for the iPhone to help users keep track of blood glucose levels on a daily basis, while the application allows patients to send selected data to their physicians to aid in monitoring their progress.10
Since there are many other blood glucose monitors available on the market, how is the iBGStar different, apart from its smartphone capabilities? The new monitor uses a novel patented technology called dynamic electrochemistry.10 Dynamic electrochemistry is a technology that uses complex mathematical methods to calculate and adjust for interference that may be caused by changes in temperature, humidity, and hematocrit levels.12 The device sends out signals of different frequencies and voltage in order to compensate for the interference that may cause inconsistent blood glucose readings.12 In patients who have abnormal hematocrit levels, which may be due to a disease state, a low hematocrit level may artificially overestimate actual blood glucose levels.12 This may pose a safety concern to the patient because it may lead him or her to use a higher insulin dose than required, possibly resulting in hypoglycemia and even hospitalization. Therefore, it is important to use a device that can measure blood glucose levels precisely in various conditions.
In order to establish the accuracy of the iBGStar, a comparison study evaluated the BGStar, a device using the same dynamic electrochemistry method, against 12 other glucose monitoring systems from various manufacturers.12 The study specifically observed the consistency of blood glucose readings with varied hematocrit concentrations. The results showed that only four of the 13 devices—the BGStar, OneTouch Verio, Glucocard G+, and Contour—actually met the study criteria for having less than 10% maximal mean percentage deviation (MMPD) from control glucose readings.12 In addition, another study has supported the accuracy of the device by showing that the iBGStar has 99.5% accuracy.13
The iBGStar is an excellent device that will provide consistent blood glucose readings and is easier to use than conventional monitors due to portability and compatibility with smartphones, but it still requires the use of needles, which may hinder compliance to glucose monitoring for some patients. In order to alleviate the discomfort associated with needles, there are several other devices currently in development that may provide needleless blood glucose monitoring.
Noninvasive Products
Few patients with diabetes monitor their blood glucose as recommended by their physician due to inconvenience and pain associated with lancing their fingers. Therefore, in order to increase the compliance of glucose monitoring, noninvasive methods are being developed. Noninvasive products could revolutionize diabetes treatment by giving patients the flexibility to check blood glucose levels more frequently, ultimately reducing morbidity and mortality secondary to uncontrolled diabetes. In addition, the novel noninvasive technologies could reduce overall health care costs by reducing hospitalizations associated with long-term diabetes complications.14
EyeSense: EyeSense is a noninvasive technology currently in development that measures blood glucose concentrations simply by placement of the measurement device near the eye.15 This innovative, noninvasive technology utilizes a novel biochemical sensor that is inserted below the conjunctiva in a simple and painless procedure by the ophthalmologist on an annual basis. The technology would replace conventional fingersticking and would probably increase blood glucose monitoring compliance. The methodology hinges on a biochemical sensor that is embedded on a small, hydrogel disk. The chemical in the disk reacts with blood glucose in the interstitial fluid below the conjunctiva of the eye and emits fluorescent light that is quantified by the photometer device. The photo-meter can be placed in front of the eye to obtain the blood glucose results in less than 20 seconds.15
The advantage of noninvasive technology is that patients have the ability to measure their blood glucose as frequently as they want without having to lance their fingers. The implanted disk is invisible to the naked eye. Additionally, it is generally well tolerated and does not feel like a foreign body in the eye of the user.15 EyeSense is still in the advanced stages of development and its approval appears promising. Projections are for a release by 2013.15
OrSense’s NBM-200G: This new, noninvasive continuous blood glucose monitoring system, which has already been approved in Europe, measures oxygen saturation, hemoglobin, and blood glucose with very high sensitivity.16 NBM-200G utilizes occlusion spectroscopy technology that correlates blood glucose levels with light-absorption and scattering measurements. The device is “operated by placing a ring-shaped probe around the patient’s finger, which applies a gentle pressure to the finger, similar to that applied during noninvasive blood-pressure measurement, and temporarily occludes the blood flow. During the occlusion, optical elements in the sensor perform a sensitive measurement of the light transmitted through the finger.”16
In a recent trial of the NBM-200G, 130,000 glucose-paired readings were taken from 450 patients to determine the accuracy of the device compared to invasive products.17 There was a strong correlation between measurements derived from the NBM-200G and those from invasive measurements. This method is painless as well as accurate in comparison to invasive devices. Therefore, it is likely to improve compliance in patients who tend to avoid fingersticks and are unable to control their diabetes with invasive products. According to OrSense, the NBM-200G is Conformité Européenne (CE) approved for noninvasive continuous monitoring in patients with demanding need for glycemic control, such as those with brittle diabetes, nocturnal hypoglycemia, and gestational diabetes.16
Conclusion
Daily monitoring of blood glucose is a very important consideration for patients with diabetes. Because hyperglycemia is usually an asymptomatic condition, patients do not feel an urgency to consistently monitor their blood sugar levels. Furthermore, SMBG is often a daunting task that, coupled with insulin injections, can be a very irritating process in the management of diabetes. The American Diabetes Association recommends that patients check their blood glucose levels at least three times daily if using insulin injections.18 It is one of the most important steps patients can take to control their diabetes; therefore, manufacturers have been diligently conceiving new products to make the process of SMBG more comfortable. Due to our busy lifestyles, devices like the iBGStar are perfect for people who are constantly on the move. It is compact, portable, and versatile, and it synchronizes with the iPhone/iPod to keep track of daily blood glucose readings. In addition, the device compensates for variability in humidity, temperature, and hematocrit, making this an ideal device for patients who travel or have blood disorders leading to abnormal hematocrit levels.
The FreeStyle InsuLinx is another advanced monitor able to perform carbohydrate calculations to aid patients in adjusting their insulin dose after a meal. The device can also connect to a Mac or PC for transferring reports, and, as with the iBGStar, the reports can be sent to a physician. Unlike the iBGStar, however, the FreeStyle InsuLinx cannot connect to a smartphone and does not have dynamic electrochemistry technology. The Freestyle InsuLinx also requires invasive monitoring of blood glucose, much like the iBGStar.
Finally, for patients who would prefer to avoid needles, a variety of noninvasive devices are emerging such as the EyeSense and NBM-200G by OrSense. While both are noninvasive, the EyeSense reads blood glucose levels via a sensor placed in the eye and the NBM-200G uses a device on the patient’s finger to read glucose levels in the blood by temporarily halting blood flow. As devices become smaller and more comfortable, patients can take advantage of cutting-edge technology to fit their lifestyle and improve diabetes control.
REFERENCES
1. National Diabetes Information Clearinghouse. www.diabetes.niddk.nih.gov. Accessed January 28, 2012.
2. Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053.
3. Burge MR. Lack of compliance with home blood glucose monitoring predicts hospitalization in diabetes. Diabetes Care. 2001;24:1502-1503.
4. Asuncion MM, Shaheen M, Ganesan K, et al. Increase in hypoglycemic admissions: California hospital discharge data. Ethn Dis. 2007;17:536-540.
5. Cavanaugh K, Huizinga MM, Wallston KA, et al. Association of numeracy and diabetes control. Ann Intern Med. 2008;148:737-746.
6. Gregory M, Adrienne Turner, Ventura T. Abbott’s new
FreeStyle InsuLinx Blood Glucose Monitoring System with insulin
calculator receives CE mark. Abbott news. May 10, 2011.
www.freestyleinsulinxmediakit.com/documents/pdf/ABT-ADC_FSI_CE_Mark_Approval_5-10-11.pdf. Accessed January 28, 2012.
7. FreeStyle InsuLinx. Abbott Diabetes Care.
www.abbottdiabetescare.co.uk/your-products/freestyle-insulinx. Accessed
January 29, 2012.
8. Abbott’s new Freestyle InsuLinx Blood Glucose
Monitoring System receives FDA clearance. Abbott Diabetes Care. March 8,
2012.
www.abbottdiabetescare.com/freestyle-insulinx-system-receives-fda-clearance.html.
Accessed April 16, 2012.
9. Apple leads mobile handsets in Q4 2011, but Android attracts more first-time smartphone buyers. February 6, 2012. The NPD Group. https://www.npd.com/wps/portal/npd/us/news/pressreleases/pr_120206. Accessed February 12, 2012.
10. iBGStar Blood Glucose Monitoring System receives U.S.
FDA 510(k) clearance. Sanofi press statements. December 7, 2011.
http://sanofi.mediaroom.com/index.php?s=64&item=57. Accessed April
16, 2012.
11. The iBGStar Blood Glucose Monitoring Device. Sanofi Canada. www.starsystem.sanofi.ca. Accessed April 12, 2012.
12. Musholt P, Schipper C, Thomé N, et al. Dynamic
electrochemistry corrects for hematocrit interference on blood glucose
determinations with patient self-measurement devices. J Diabetes Sci Technol. 2011;5:1167-1175.
13. Mitri M, Sachsenheimer D, Borchert M, et al. Clinical
accuracy of the patient self-testing blood glucose meters BGStar and
iBGStar. Institute for Clinical Research and Development. www.ikfe.de.
Accessed February 13, 2012.
14. Amir O, Weinstein D, Zilberman S. Continuous noninvasive glucose monitoring technology based on “occlusion spectroscopy.” J Diabetes Sci Technol. 2007;1:463-469.
15. About us. EyeSense. www.eyesense.com/en/ueber.htm. Accessed February 10, 2012.
16. Glucose. Diabetes clinical overview. OrSense. www.orsense.com/?id=812. Accessed February 13, 2012.
17. Clinical trials. Blood glucose monitoring. OrSense. www.orsense.com/?id=815. Accessed April 10, 2012.
18. American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(suppl 1):S11-S63.
To comment on this article, contact rdavidson@uspharmacist.com.





