US Pharm. 2013;38(5)(Oncology suppl):3-7.
ABSTRACT: Cancer patients who develop infections, especially those receiving chemotherapy, need to seek immediate medical attention and possibly be hospitalized. Optimal management of patients at risk for febrile neutropenia (FN) includes immediate risk assessment, evaluation of any drug allergies, and obtaining appropriate culture specimens, followed by prompt initiation of empirical broad-spectrum IV antibiotics and careful monitoring in those with previously documented FN. Bone marrow suppression and its complications, including FN, often represent major dose-limiting toxicities associated with systemic cancer chemotherapy that can result in considerable morbidity, mortality, and costs. Efforts to reduce the risk of FN should be based on appropriate antimicrobial stewardship and the prophylactic use of myeloid growth factors where indicated.
Febrile neutropenia (FN) infections in cancer patients receiving chemotherapy should be considered medical emergencies requiring immediate medical evaluation and administration of empirical broad-spectrum antibiotics.1 Clinical studies demonstrate that patients with FN not promptly treated often experience a rapidly fatal outcome, most notably because of gram-negative bacteremia, with reported rates of mortality of 5% to 20% that increase in direct proportion to the number of major infectious complications and comorbid medical conditions.2-4 In fact, mortality in excess of 50% despite prompt antibiotic treatment has been reported in neutropenic patients presenting with septic shock, pneumonia, and documented bacteremia.4-13 Although there have been improvements in clinical treatment of infections, FN remains a major cause of morbidity, mortality, and cost among patients with cancer and may limit the delivery of full-dose chemotherapy on schedule.4,11,13,15
Pathophysiology
Cancer patients receiving cytotoxic antineoplastic therapy sufficient to adversely affect myelopoiesis and the integrity of the gastrointestinal (GI) mucosa are at greater risk for invasive infection due to colonizing bacteria or fungi that translocate across intestinal mucosal surfaces.14-21 The most common source of infection in these patients is often self-infection with normal body flora that may include gram-negative and gram-positive bacteria, viruses, and/or fungi.10,11
Neutropenia can occur without any observable clinical complications, meaning neutrophil counts can drop low and then return to a normal level without typical signs of infection (TABLE 1). However, a low neutrophil count increases the risk of developing an infection even when usual infection prevention methods are utilized (TABLE 2). Fever in these patients is usually defined as a single temperature of 38.3°C or a temperature of 38°C sustained for 1 hour without an obvious cause.8,10,11,18-20 Fever is actually caused by infection in 50% to 70% of cases, and bacteremia may be present in as many as 20% to 30% of all cases.8,10,11,18

Neutropenia is defined as an absolute neutrophil count (ANC) <500 neutrophils/mcL or an ANC <1,000/mcL with an expected decline to 500/mcL or less in 48 hours. There are many factors that contribute to the development of neutropenia, including dosage and duration of chemotherapy (a higher dosage usually causes a lower neutrophil count); type of chemotherapy (some chemotherapies cause a greater drop in neutrophils than others); and whether or not patients have other medical problems in addition to cancer, such as HIV infection, or take other immunosuppressive therapy.21-23
The diagnosis of infection in neutropenic patients is complicated because there is generally a lack of elevated white blood cells (WBCs) or left shift.10,11 Usual signs and symptoms of infection in typical patients, such as pus, abscesses, and infiltrates on chest x-rays, depend on the presence of WBCs and a healthy immune system response, and these are often attenuated in cancer patients. The most reliable indication of infection in neutropenic patients is often an elevated temperature, but severe infections can occur in the absence of fever or neutropenia. Definitive cultures may take days, and a septic neutropenic cancer patient may die in a few hours if not hospitalized and empirically treated with IV antibiotics.10,11
Seeding of the bloodstream by endogenous bacteria from the GI tract is felt to be responsible for the majority of cases of FN.10,11 Many chemotherapy drugs can have an adverse effect on the mucosal barrier (i.e., can cause mucositis). Blood cultures are positive in about 20% to 30% of cases.1,16,18 Gram-positive organisms are isolated more commonly than gram-negative organisms, but the latter are associated with more severe infections, including sepsis.

Neutropenic Patient Management and Clinical Evaluation
Cancer patients who develop infections need to immediately seek medical attention and receive appropriate treatment. Optimal management of patients at risk for FN includes evaluation to identify any drug allergies and obtaining appropriate cultures followed by prompt initiation of appropriate empirical broad-spectrum antibiotics, and careful monitoring in those with documented FN.1,17,18 Patient evaluation should include drug allergies; prior antibiotic history; clinical picture; local flora; a complete medication profile and medical history; and physical examination with particular attention directed to the oropharynx and periodontium, sites of catheters, recent procedures, the perineum area, nail beds, nares, and external auditory canals.20,21 The assessment should also include mental status; hydration status; condition of oral and pharyngeal mucosa and skin, including any indwelling IV sites; respiratory system; abdomen; and cardiovascular system including signs of sepsis. Special considerations include the possibility of other infections such as meningitis, sinusitis, herpes simplex or herpes zoster, and thrush. Due to endogenous bacteria in the GI tract, rectal exams should be avoided, but perirectal inspection for abscesses should be done.
Clinical laboratory tests needed should include the following16,17,20: a complete hematologic profile or complete blood cell count (CBC) with differential; blood chemistry profile; blood cultures (for aerobic and anaerobic organisms) and cultures from any indwelling IV lines from all potential sites, along with at least two sets of blood cultures from peripheral veins or one set each from a peripheral vein and a central venous catheter; urinalysis; urine culture (note that the absence of pus cells on urinalysis does not rule out a urinary tract infection in the setting of neutropenia); and sputum gram stain and culture if sputum is productive. Although a specific organism may only be found in about half of patients with fever and neutropenia, a presumptive diagnosis of infection is important because of the high risk of withholding antimicrobial therapy.
Chest radiographs should be obtained when clinical evidence of a respiratory infection is present.16 Pulmonary infiltrates may not develop until the neutropenia begins to recover. A CT scan of the chest may be helpful to substantiate signs of fungal infection in high-risk patients with unexplained fever who have not responded to 3 to 5 days of antibacterial therapy.22
Risk Assessment for Febrile Neutropenic Patients
The risk of FN increases in direct proportion to the severity and duration of neutropenia and occurs most frequently early in a course of chemotherapy. Early neutropenic events often prompt chemotherapy dose reductions or delays or the addition of prophylactic myeloid growth factors or antibiotics in subsequent treatment cycles.14-16 Algorithmic patient-risk assessment approaches developed over the past 40 years are often utilized to help diagnose and treat fever and neutropenia, and provide infection prophylaxis as well. These algorithms have guided and modified clinical evidence and experience over time. An example of this approach is the Infectious Disease Society of America (IDSA) Fever and Neutropenia Guidelines. These guidelines define risk assessment for high- and low-risk patients with fever and neutropenia as follows11:
“1. Assessment of risk for complications of severe infection should be undertaken at presentation of fever (A-II). Risk assessment may determine the type of empirical antibiotic therapy (oral vs. intravenous [IV]), venue of treatment (inpatient vs. outpatient), and duration of antibiotic therapy (A-II).”
2. High-risk patients are defined as “those with anticipated prolonged (>7 days duration) and profound neutropenia (absolute neutrophil count [ANC] ≤100 cells/mm3 following cytotoxic chemotherapy) and/or significant medical comorbid conditions, including hypotension, pneumonia, new-onset abdominal pain, or neurologic changes. Such patients should be initially admitted to the hospital for empirical therapy (A-II).”
“3. Low-risk patients, including those with anticipated brief (<7 days duration) neutropenic periods or no or few comorbidities, are candidates for oral empirical therapy (A-II).”
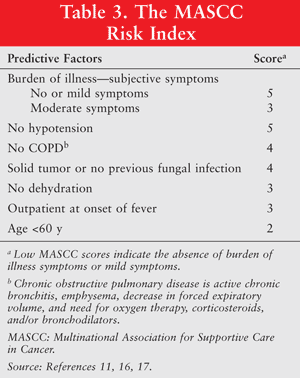
“4. Risk classification may be performed using the Multinational Association for Supportive Care in Cancer (MASCC) scoring system (B-I). i. High-risk patients have a MASCC score <21 (B-I). All patients at high risk by MASCC or by clinical criteria should be initially admitted to the hospital for empirical antibiotic therapy if they are not already inpatients (B-I). ii. Low-risk patients have a MASCC score ≥21 (B-I). Carefully selected low-risk patients may be candidates for oral and/or outpatient empirical antibiotic therapy (B-I).”
Low MASCC scores indicate the absence of burden of illness symptoms or mild symptoms (TABLE 3).11,16,17 Patient evaluation and risk assessment scores help clinicians determine the urgency, scope, and extent of medical care needed for patients with FN.
Empirical Antibiotic Treatment for Febrile Neutropenic Patients
Clinicians treating neutropenic patients face a daunting task of keeping up-to-date with infection risks; diagnostic methods; antimicrobial therapies required for management of febrile patients through the neutropenic period; and antibiotic-resistance patterns in the local medical community. Accordingly, the IDSA has also established algorithmic treatment approaches to assist clinicians with diagnosis and treatment of fever and neutropenia and with infection prophylaxis.11
Gram-positive organisms are often the predominant bacterial pathogens identified, with coagulase-negative staphylococci, Staphylococcus aureus, Enterococcus species (spp), and viridans group streptococci being isolated most often.11,24,25 Among gram-negative bacilli, Escherichia coli, Klebsiella spp, and Pseudomonas aeruginosa remain the predominant pathogens, although other Enterobacteriaceae, Stenotrophomonas maltophilia, and Acinetobacter spp are isolated frequently.26,27 Approximately 10% to 15% of bacteremia is polymicrobial, with gram-negative bacilli being isolated from more than 80% of these infections.28-30
Resistance to common antimicrobial agents is being encountered increasingly at most hospitals, in part because of heavy use of antibiotics.31 Of particular concern, more than 50% of S aureus isolates are methicillin-resistant S aureus (MRSA), and vancomycin resistance among enterococci characterizes nearly 30% of enterococcal isolates nationwide.31,32 Additionally, reduced susceptibility or resistance to penicillin is being reported increasingly with the viridans group streptococci and in Streptococcus pneumoniae isolates.31,32 Invasive fungal infections are most often seen in patients with prolonged neutropenia and after stem cell trans-plantation.33 Empirical broad-spectrum antibiotic therapy should be administered promptly to all patients with FN.1
Patient risk should be quickly assessed to determine the number and spectrum of the antibiotic regimen (monotherapy or combination therapy), route of administration (parenteral or oral), and location of treatment (hospital vs. outpatient).33,34 Low-risk patients may be defined as ambulatory patients with good performance status and no serious comorbidity and an anticipated short duration of severe neutropenia or as those with an index score of 21 or more using the predictive model developed and validated by the MASCC score (TABLE 3).11,16,17
The development of reasonably accurate risk prediction rules and the availability of suitable antimicrobial agents, ambulatory centers, and evidence of safety and efficacy have made the treatment of low-risk patients with FN in the ambulatory setting feasible.35,36 Such an approach requires appropriate infrastructure and a multidisciplinary team of health care providers. Patients managed in the ambulatory setting should be tracked for readmission, important complications, or mortality. Many clinicians prefer an initial 24- to 48-hour period of hospitalization for evaluation and initiation of empirical broad-spectrum antibiotics followed by outpatient treatment for the duration of the neutropenic episode.37,38
Although there is little to distinguish the many empirical regimens that have been studied in randomized, controlled trials, many clinicians agree that patients at higher risk should be hospitalized immediately to receive parenteral antibiotic therapy.1 Monotherapy with an antipseudomonal beta-lactam antibiotic, such as an extended spectrum cephalosporin or a carbapenem, is often adequate treatment for uncomplicated episodes. Combination regimens with a beta-lactam and either an aminoglycoside or a fluoroquinolone should be considered in the treatment of complicated infections, including those with pneumonia, neutropenic enterocolitis, or the presence of hypotension or shock. Myeloid growth factors are not recommended routinely in the treatment of established FN, although they may be useful in patients at increased risk for serious complications including mortality.16,39
Patients who defervesce rapidly without identification of a specific pathogen should be continued on antibiotics through the period of neutrophil recovery.1 When a specific organism has been identified, patients should be treated for 7 to 14 days. Vancomycin should be considered with catheter-related infection, gram-positive organisms on blood culture, hypotension, mucosal damage, or colonization with penicillin-resistant pneumococci or MRSA.1 For neutropenic patients with persistent fever after 3 to 5 days, empirical antifungal therapy may be considered.1
Prevention of Febrile Neutropenia
Strategies aimed at reducing the risk and consequences of FN should include appropriate antimicrobial stewardship, with the task of effectively treating infections so as to significantly limit the emergence of resistant microorganisms while preserving the utility of available antimicrobial agents.40,41 Prophylactic antibiotics have demonstrated some efficacy in reducing the risk of febrile episodes in neutropenic patients with cancer; however, these agents have been associated with additional toxicity and the emergence of antibiotic-resistant bacteria.1,42 Although they can be potentially efficacious in high-risk patients and in those who develop serious neutropenic complications despite hematopoietic growth factor support, the IDSA and the American Society of Clinical Oncology (ASCO) recommend against routine antibacterial prophylaxis.1,16
Myeloid growth factors administered prophylactically after cancer chemotherapy but before the onset of neutropenia have been shown to reduce the risk of FN and documented infection.43 A meta-analysis of randomized, controlled trials of granulocyte colony-stimulating factor (G-CSF) has also demonstrated a significant reduction in infection-related and early all-cause mortality while improving delivery of chemotherapy dose intensity.44
Current ASCO guidelines recommend primary prophylaxis with a myeloid growth factor in patients with cancer who are receiving chemotherapy associated with a 20% or greater risk of FN, as well as in those with certain high-risk factors such as profound neutropenia (ANC <100) anticipated to extend 0.7 days; hemodynamic instability; oral or GI mucositis that interferes with swallowing or causes severe diarrhea; GI symptoms including abdominal pain, nausea, and vomiting or diarrhea; neurologic or mental status change of new onset; intravascular catheter infection, especially catheter tunnel infection; new pulmonary infiltrates or hypoxemia or underlying chronic lung disease; and evidence of hepatic or renal insufficiency.16,17,45 High-risk patients require hospitalization for IV empirical antibiotic therapy such as monotherapy with an antipseudomonal beta-lactam agent (e.g., cefepime), a carbapenem (meropenem or imipenem-cilastin), or piperacillin-tazobactam.45
Conclusion
Myelosuppression and its complications, including FN, continue to represent major dose-limiting toxicities associated with systemic cancer chemotherapy that often result in considerable morbidity, mortality, and costs. The management of FN requires immediate evaluation, risk assessment, and treatment with empirical broad-spectrum antibiotics. Efforts to reduce the risk of FN should be based on appropriate antimicrobial stewardship and the prophylactic use of myeloid growth factors where indicated.
REFERENCES
1. Hughes WT, Armstrong D, Bodey GP, et al. 2002
guidelines for the use of antimicrobial agents in neutropenic patients
with cancer. Clin Infect Dis. 2002;34:730-751.
2. Bodey GP, Buckley M, Sathe YS, et al. Quantative
relationships between circulating leukocytes and infection in patients
with acute leukemia. Ann Intern Med. 1966;64:328-340.
3. Paul M, Yahav D, Fraser A, et al. Empirical antibiotic
monotherapy for febrile neutropenia: systematic review and meta-analysis
of randomized controlled trials. J Antimicrob Chemother. 2006;57:176-189.
4. Kuderer NM, Dale DC, Crawford J, et al. Mortality,
morbidity, and cost associated with febrile neutropenia in adult cancer
patients. Cancer. 2006;106:2258-2266.
5. González-Barca E, Fernández-Sevilla A, Carratalá J, et
al. Prognostic factors influencing mortality in cancer patients with
neutropenia and bacteremia. Eur J Clin Microbiol Infect Dis. 1999;18:539-544.
6. Malik I, Hussain M, Yousuf H. Clinical characteristics
and therapeutic outcome of patients with febrile neutropenia who present
in shock: need for better strategies. J Infect. 2001;42:120-125.
7. Darmon M, Azoulay E, Alberti C, et al. Impact of
neutropenia duration on short-term mortality in neutropenic critically
ill cancer patients. Intensive Care Med. 2002;28:1775-1780.
8. Carratalà J, Rosón B, Fernández-Sevilla A, et al.
Bacteremic pneumonia in neutropenic patients with cancer: causes,
empirical antibiotic therapy, and outcome. Arch Intern Med. 1998;158:868-872.
9. Dellinger RP, Levy MM, Rhodes A, et al. Surviving
sepsis campaign: international guidelines for management of severe
sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637.
10. Schiel X, Hebart H, Kern WV, et al. Sepsis in
neutropenia: guidelines of the Infectious Diseases Working Party (AGIHO)
of the German Society of Hematology and Oncology (DGHO). Ann Hematol. 2003;82(suppl 2):S158-S166.
11. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical
practice guideline for the use of antimicrobial agents in neutropenic
patients with cancer: 2010 update by the Infectious Diseases Society of
America. Clin Infect Dis. 2011;52:e56-e93.
12. Wisplinghoff H, Seifert H, Wenzel RP, et al. Current
trends in the epidemiology of nosocomial bloodstream infections in
patients with hematological malignancies and solid neoplasms in
hospitals in the United States. Clin Infect Dis. 2003:36:1103-1110.
13. Elting LS, Rubenstein EB, Rolston KV, et al. Outcomes
of bacteremia in patients with cancer and neutropenia: observations from
two decades of epidemiological and clinical trials. Clin Infect Dis. 1997;25:247-259.
14. Amgen Fact Sheets: Hematology. Thousand Oaks, CA: Amgen; 2006.
15. Aapro MS, Link H. September 2007 update on EORTC guidelines and anemia management with erythropoiesis-stimulating agents. Oncologist. 2008;13:33-36.
16. Lyman GH, Rolston KV. How we treat febrile neutropenia in patients receiving cancer chemo-therapy. J Oncol Pract. 2010;6:149-152.
17. Klatersky J, Paesmans M, Rubenstein EB, et al. The
Multinational Association for Supportive Care in Cancer risk index: a
multinational scoring system for identifying low-risk febrile
neutropenic cancer patients. J Clin Oncol. 2000;18:3038-3051.
18. Bow E. Treatment and prevention of neutropenic fever syndromes in adult cancer patients at low risk for complications. UpToDate.
www.uptodate.com/contents/treatment-and-prevention-of-neutropenic-fever-syndromes-in-adult-cancer-patients-at-low-risk-for-complications.
Accessed February 1, 2013.
19. Wingard JR. Treatment of neutropenic fever syndromes
in adults with hematologic malignancies and hematopoietic cell
transplant recipients (high risk patients). UpToDate.
www.uptodate.com/contents/treatment-of-neutropenic-fever-syndromes-in-adults-with-hematologic-malignancies-and-hematopoietic-cell-transplant-recipients-high-risk-patients/abstract/50.
Accessed February 1, 2013.
20. Sickles EA, Greene WH, Wiernik PH. Clinical presentation of infection in granulocytopenic patients. Arch Intern Med. 1975;135:715.
21. Kusick K. The use of colony-stimulating factors with myelosuppressive chemotherapy. Cleveland Clin Pharmacother Update. 2009;12:1-5. www.clevelandclinicmeded.com/medicalpubs/pharmacy/pdf/Pharmacotherapy_XII-1.pdf. Accessed October 29, 2012.
22. Caillot D, Couaillier JF, Bernard A, et al. Increasing
volume and changing characteristics of invasive pulmonary aspergillosis
on sequential throracic computed tomography scans in patients with
neutropenia. J Clin Oncol. 2001;19:253-259.
23. Brown CH. Bone marrow modulators: colony-stimulating factors and erythrocyte-stimulating agents. US Pharm. 2013;38(1)(Oncology suppl):3-7.
24. Wispilinghoff H, Seifert H, Wenzel RP, et al. Current
trends in the epidemiology of nosocomial bloodstream infections in
patients with hematological malignancies and solid neoplasms in
hospitals in the United States. Clin Infect Dis. 2003;36:1103-1110.
25. Yadegarynia D, Tarrand J, Raad I, et al. Current spectrum of bacterial infections in patients with cancer. Clin Infect Dis. 2003;37:1144-1145.
26. Rolston KV, Yadegarynia D, Kontoyiannis DP, et al. The
spectrum of gram-positive blood-stream infections in patients with
hematologic malignancies, and the in vitro activity of various
quinolones against Gram-positive bacteria isolated from cancer patients.
Int J Infect Dis. 2006;45:223-230.
27. Safdar A, Rolston KV. Immunocompromised hosts: Stenotrophomonas maltophilia: changing spectrum of a serious bacterial pathogen in patients with cancer. Clin Infect Dis. 2007;45:1602-1609.
28. Rolston KV, Bodey GP, Safdar A, et al. Polymicrobial
infection in patients with cancer: an underappreciated and underreported
entity. Clin Infect Dis. 2007;45:228-233.
29. Klastersky J, Ameye L, Maertens J, et al. Bacteraemia in febrile neutropenic cancer patients. Int J Antimicrob Agents. 2007;30(suppl 1):S51-S59.
30. Rolston KV. Challenges in the treatment of infections
caused by gram-positive and gram-negative bacteria in patients with
cancer and neutropenia. Clin Infect Dis. 2005;40(suppl 4):S246-S252.
31. Han XY, Kamana M, Rolston KV. Viridans streptococci
isolated by culture from blood of cancer patients: clinical and
microbiologic analysis of 50 cases. J Microbiol. 2006;30:160-165.
32. Kumashi P, Girgawy E, Tarrand JJ, et al. Streptococcus pneumoniae bacteremia in patients with cancer: disease characteristics and outcomes in the era of escalating drug resistance (1998-2002). Medicine (Baltimore). 2005;84:303-312.
33. Cornely OA, Maertens J, Winston DJ, et al.
Posaconazole vs. fluconazole or itraconazole prophylaxis in patients
with neutropenia. N Engl J Med. 2007;356:348-359.
34. Talcott JA, Siegel RD, Finberg R, et al. Risk
assessment in cancer patients with fever and neutropenia: a prospective,
two-center validation of a prediction rule. J Clin Oncol. 1992;10:316-322.
35. Kern WV. Risk assessment and treatment of low-risk patients with febrile neutropenia. Clin Infect Dis. 2006:42:533-540.
36. Rolston KV, Frisbee-Hume SE, Patel S, et al. Oral
moxifloxacin for outpatient treatment of low-risk, febrile neutropenic
patients. Support Care Cancer. 2010;18:89-94.
37. Klastersky J, Paesmans M, Georgala A, et al.
Outpatient oral antibiotics for febrile neutropenic cancer patients
using a score predictive for complications. J Clin Oncol. 2006;24:4129-4134.
38. Sung L, Nathan PC, Lange B, et al. Prophylactic
granulocyte colony-stimulating factor and granulocyte-macrophage
colony-stimulating factor decrease febrile neutropenia after
chemo-therapy in children with cancer: a meta-analysis of randomized
controlled trials. J Clin Oncol. 2004;22:3350-3356.
39. Clark OA, Lyman GH, Castro AA, et al.
Colony-stimulating factors for chemotherapy-induced febrile neutropenia:
a meta-analysis of randomized controlled trials. J Clin Oncol. 2005;23:4198-4214.
40. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious
Diseases Society of America and the Society for Healthcare Epidemiology
of America guidelines for developing an institutional program to enhance
antimicrobial stewardship. Clin Infect Dis. 2007;44:159-177.
41. Moellering RC Jr, Graybill JR, McGowan JE Jr, et al.
Antimicrobial resistance prevention initiative—an update: proceedings of
an expert panel on resistance. Am J Med. 2007;120:S4-S28.
42. Bucaneve G, Micozzi A, Menichetti F, et al.
Levofloxacin to prevent bacterial infection in patients with cancer and
neutropenia. N Engl J Med. 2005;353:977-987.
43. Crawford J, Ozer H, Stoller R, et al. Reduction by
granulocyte colony-stimulating factor of fever and neutropenia induced
by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164-170.
44. Kuderer NM, Dale DC, Crawford J, et al. Impact of
primary prophylaxis with granulocyte colony-stimulating factor on
febrile neutropenia and mortality in adult cancer patients receiving
chemotherapy: a systematic review. J Clin Oncol. 2007;25:3158-3167.
45. Lyman GH, Kleiner JM. Summary and comparison of myeloid growth factor guidelines in patients receiving cancer chemotherapy. J Natl Compr Canc Netw. 2007;5:217-228.
To comment on this article, contact rdavidson@uspharmacist.com.





