ABSTRACT: Clostridium difficile is a common entity causing healthcare-associated diarrhea, the primary pathogen for antibiotic-associated colitis, and an important cause of infectious disease–associated mortality in the United States. Cases of recurrent C difficile infection are increasing in both number and severity. Fecal microbiota transplantation (FMT) is an alternative treatment for patients with C difficile infection refractory to anti-infective therapy. Although FMT is proven effective, the preferred route of administration and long-term side effects of this treatment, as well as its application for other disease states, still require further research.
US Pharm. 2016;41(4)HS-22-HS-26.
Within the healthcare setting, the bacterium Clostridium difficile has been identified as the most common cause of infectious diarrhea.1 After initial exposure to the pathogen, patients are at an increased risk for recurrent infections. The financial burden on the healthcare system resulting from additional costs, either during the initial episode or from recurrent infections, should be taken into consideration. Thus, patients who present with recurrent infections or for whom pharmacologic treatment has not been successful should be considered for fecal microbiota transplantation (FMT). Pharmacists can play a significant role in assisting with the pharmacotherapy management of these patients and aid in identifying appropriate patients who have experienced multiple C difficile infection (CDI) relapses despite standard therapy.
Pathophysiology of C difficile
C difficile has been recognized as the primary pathogen responsible for antibiotic-associated colitis and is the causative agent for 15% to 25% of cases of nosocomial antibiotic-associated diarrhea.1 According to the CDC, more than 250,000 Americans each year require hospitalization for CDI and roughly 14,000 of these cases are fatal.2 In its 2013 report on drug resistance, the CDC classified C difficile as an “urgent public health threat.”2 CDI has been identified primarily as a hospital-acquired infection that has been associated with prolonged hospital length of stay, and a significant contributor to morbidity and mortality.3
C difficile is an anaerobic gram-positive bacterium first described in 1935 that is transmitted from one individual to another via the fecal-oral route.1,3,4 In the spore form, C difficile is resistant to gastric acid and is capable of passing through the stomach into the intestines.3 This allows the bacterium to change to the vegetative life cycle where it releases the potent exotoxins A and B, thus causing the colitis symptoms seen in many patients.3 The disease may be carried asymptomatically, or it may present with clinically significant symptoms ranging from mild-to-severe diarrhea to pseudomembranous colitis.
CDI commonly occurs in response to alteration of normal gut flora. This is usually associated with systemic antibiotic use.3 Almost every class of antimicrobials has been implicated in the occurrence of CDI, but clindamycin, third-generation cephalosporins, extended-spectrum penicillins, and fluoroquinolones appear to be the major causative agents.1,3,4
Standard-of-Care Therapies
Treatment options for CDI range from oral antibiotics to surgery, depending upon the severity of infection. Treatment of CDI should begin with prompt diagnosis, discontinuation of the provoking agent(s), if possible, and initiation of effective therapy.3 Oral metronidazole 500 mg three times daily for 10 to14 days is indicated for mild-to-moderate CDI, including both the initial episode and the first recurrence.1 Oral vancomycin 125 mg four times per day for 10 to14 days is recommended therapy for an initial CDI episode categorized as severe or for a first CDI recurrence. Oral vancomycin is also first-line therapy when treating a second CDI recurrence.1 Although metronidazole and vancomycin have been found to have comparable efficacy results for mild-to-moderate CDI in published prospective, randomized, controlled trials, metronidazole is indicated as first-line therapy owing to its relatively lower cost.1,3,5 Additionally, oral vancomycin is indicated for those patients who are intolerant to metronidazole, who fail to respond to metronidazole, or who have severe cases of CDI.1,3-5 For severe, complicated CDI, categorized by the presence of hypotension or shock, ileus, or megacolon, oral vancomycin 500 mg four times per day (per rectum if ileus is present), with or without metronidazole, is recommended.1 Oral fidaxomicin 200 mg twice daily was FDA-approved for CDI treatment in 2011 and has been shown to be noninferior to vancomycin.6 Fidaxomicin has also shown benefit in reducing the development of recurrent CDI episodes; however, this appears to be isolated to non-NAP1/BI/027 C difficile strains.4,6 Medications that do not carry FDA approval but have been used off-label for CDI include rifaximin and nitazoxanide. With the use of rifaximin, caution is advised owing to the risk that certain strains of C difficile will develop resistance during therapy.1
Fecal Microbiota Transplantation
As the number of documented cases of recurrent CDI increases, the need for alternative methods of therapy becomes more and more apparent. From 1996 to 2003, the number of diagnosed cases of CDI doubled from 31/100,000 to 61/100,000.7 After a first occurrence of CDI, patients are at an increased risk of a recurrent episode. Approximately 15% to 30% of patients with a documented case of CDI have been found to experience a recurrent episode; subsequent recurrences have developed in approximately 65% of these patients.7,8 Recurrent infections can be difficult to manage, which has led physicians to explore alternative treatment options. One therapeutic option gaining increased scientific support and use in practice is FMT therapy.
FMT for the treatment of CDI dates back to the 1983 work of Schwan et al; over the past 2 to 3 years there has been an increase in the published literature describing its successful use.9 FMT is thought to restore the balance of normal commensal organisms in the colon via donor feces, ultimately reducing the ability of C difficile to thrive.8,10 FMT is growing in popularity for the treatment of recurrent CDI, owing to the direct approach of restoring the normal intestinal flora via donor feces.10 In a multicenter, long-term follow-up study of patients undergoing colonoscopic FMT, 97% of patients indicated they would undergo the same process if they experienced another case of recurrent CDI.11
FMT Indications
In 2010, a work group was formed to assist in determining the appropriate clinical use of FMT therapy.12 This work group developed primary indications to guide physicians on the use of FMT, listed in Table 1.7
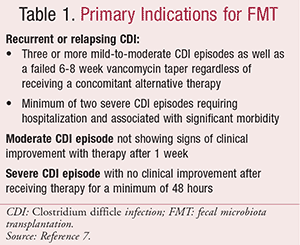
Most recently, in 2013, the American College of Gastroenterology released C difficile treatment guidelines that indicate that FMT therapy may be considered as an alternative for patients who have experienced a third recurrence of CDI after receiving vancomycin as a pulse or taper regimen.13 Each patient case should be evaluated individually to determine if FMT therapy is warranted. If antimicrobial therapy has failed for recurrent CDI, surgery is also an alternative option and may be discussed with the patient. At this time, FMT therapy has not been FDA-approved. If FMT therapy is being used for an indication other than CDI, an Investigational New Drug (IND) application is required.12 The FDA has stated that an IND application requirement will not be enforced for CDI if the following three actions are performed by the provider: 1) informed consent is obtained; 2) the patient is thoroughly informed regarding associated risk with treatment; and 3) the patient is informed that FMT is classified as an investigational treatment option.12
Donor and Recipient
There are two main sources of human feces for FMT administration: 1) a sample donated by a close family member or intimate partner; 2) an unrelated-donor sample provided through a stool bank.14 Regardless of the source of stool, general recommendations include screening for a variety of pathogens, toxins, antigens, and diseases (Table 2).7,8,10
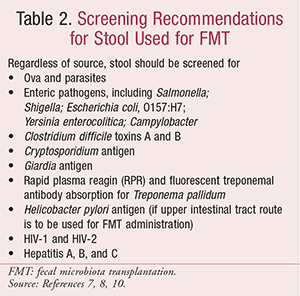
Additional guidance includes contraindications to stool donations from patients with7
Risk of an infectious agent: For example, from high-risk sexual behaviors; use of illicit drugs; tattoo/body piercing in previous 6 months; incarceration or history of incarceration; known communicable disease; travel (in last 6 months) to areas where diarrheal illnesses are endemic and risk of traveler’s diarrhea is high), or known infection with HIV or hepatitis B or C
Gastrointestinal comorbidities: History of inflammatory bowel disease, irritable bowel disease, idiopathic chronic constipation, chronic diarrhea, gastrointestinal malignancy/known polyposis
Factors affecting intestinal microbiota: Antibiotics in previous 3 months; major immunosuppressive medications (i.e., calcineurin inhibitors, exogenous glucocorticoids, biologic agents); systemic antineoplastic agents
Recent ingestion of potential allergen to which that recipient has known allergy (e.g., nuts).
FMT Procedure: Administration Considerations
The sample for instillation may be administered through the upper intestinal tract (via nasogastric or nasoduodenal tube) or the lower one (via colonoscopy or retention enema).14 Published literature and reviews indicate the most common route of instillation is the lower intestinal tract.8,14 Patients with recurrent C difficile infection who are scheduled for FMT will usually remain on treatment for C difficile infection until 1 or 2 days prior to FMT. Attention should be given to minimizing the use of other systemic antibiotics prior to FMT to maximize efficacy of the procedure.
Most protocols recommend administration of a bowel preparation the day before FMT, regardless of route of FMT instillation.7,9 Loperamide may be given the morning of FMT prior to instillation to aid in retention of the sample, but this is not required. For FMT administered via nasoduodenal tube, a proton pump inhibitor (PPI) should be given the evening prior to and the morning of FMT.7
The total volume of product ideal for FMT administration has not been established; however, smaller volumes (~50 ml) are typically used when given via nasoduodenal tube, and larger volumes (~250-300 ml) given with colonoscopic/enema administration.7 Resolution of symptoms is the primary endpoint post-FMT. It is not recommended to retest for C difficile in patients who do not have symptoms, as individuals may be colonized with C difficile.7 Antibiotic exposure should be avoided in patients who are post-FMT to sustain FMT efficacy and minimize CDI recurrence.
More recently, performing FMT via oral administration of frozen encapsulated stool has been explored. A recent report of elderly patients (aged 72-92 years) receiving FMT via this modality says they tolerated it well, with a good success rate.15 It is to be noted that some patients in this report achieved success with encapsulated FMT after failure with FMT via colonoscopy. Another published report of 19 adult patients receiving encapsulated FMT demonstrated success in 13 patients after one FMT course and in four patients who were cured after a second course; however, two patients did experience failure.16 Ultimately, the decision on route of FMT administration must be tailored to the individual patient.
FMT Benefits and Risks
FMT is used for patients who are refractory to anti-infective therapy for CDI. It is an alternative treatment modality that may offer benefit for patients who have experienced multiple episodes of CDI after exhausting anti-infective treatment options. It is also likely to be cost-effective when compared to certain anti-infective agents and hospitalization costs.4 FMT is becoming a more common practice, as additional literature indicates that patients experience successful resolution of CDI. In one study, a comparison between oral vancomycin and duodenal infusion of donor feces found that patients who received the infusion had higher resolution of CDI.17
Currently, there is no consensus on the optimal route of FMT administration owing to the lack of studies directly comparing the efficacy of colonoscopy, nasogastric tube, nasojejunal tube, nasoduodenal tube, and enemas; thus, researchers have been unable to determine the benefit of one route versus another.18 Overall, recent publication of FMT therapy studies regardless of route of administration demonstrate that rate of resolution is high. In one study, 467 out of 536 patients (87%) who received FMT experienced complete resolution of diarrhea after one transplant.19
Despite the success rate, there may be risks related to the procedure. Colonoscopy offers the ability to use larger volumes and to inspect the colon, but perforation may be a potential complication. Alternatively, the use of enemas offers a less invasive option and anesthesia is not required; however, enemas may require multiple administrations.19 The use of a nasogastric or nasojejunal tube may decrease preparation time and patient inconvenience, although aspiration may be a concern.20
Risks associated with FMT therapy also focus on transmission of an infectious agent. According to the guidelines of the Society for Healthcare Epidemiology of America (SHEA), it is recommended that the donor be screened for transmissible agents.1 There is the option to obtain unrelated donor stool from a donor stool bank, where prescreening has been performed. To date, there are no reports of infectious disease transmission through FMT administration.
Post FMT, patients may experience common side effects such as belching, nausea and vomiting, abdominal cramps, diarrhea, and constipation.17,18 These may occur the day of the FMT but are self-limiting. There may be a higher risk for adverse events in individuals who7
• Are receiving immunosuppressant agents (high-dose corticosteroids, calcineurin inhibitors, etc.)
• Have a history of immunodeficiency (bone marrow transplant, advanced HIV/AIDS, etc.)
Most of the available published literature highlights the experience of FMT in the adult population, but very little has been described in the pediatric population. Additionally, there is a lack of data regarding long-term safety risks associated with FMT. Overall, FMT appears to be safe and effective. The benefit of FMT in CDI has expanded interest and application to other disease states, such as irritable bowel syndrome.18
Conclusion
FMT has proven effective for CDI treatment, but continued research is needed to assess the benefits of each route of administration, alternate indications, and long-term side effects. Large randomized, controlled clinical trials would be beneficial in determining the impact of FMT therapy among larger, more diverse populations. These trials would aid the medical community in making informed decisions about the use of FMT in varying patient groups.
Pharmacists play a role in the management and identification of patients who are eligible for FMT. In addition, the pharmacist may assist in the procurement of the FMT product, as well as collaborate with various healthcare team members to optimize FMT efficacy. The pharmacist can also monitor for any medication-related risk factors, such as the use of PPIs and antibiotics that can promote CDI. When suggesting FMT therapy, all members of the healthcare team should take into account safety and patient preference when determining an appropriate route of administration. More research also needs to be done to determine a safe and efficient way to screen for donors, especially in acute settings. Determining the effects of FMT on quality of life, morbidity, and mortality will further aid medical providers in determining whether a patient is a candidate for therapy.
REFERENCES
1. Cohen SH, Gerding DN, Johnson S, et al:. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
2. CDC. Antibiotic resistance threats in the United States. 2013. Updated July 14, 2014. www.cdc.gov/drugresistance/threat-report-2013. Accessed October 11, 2015.
3. Fraser TG, Swiencicki JF. Clostridium difficile. February 2013. www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/infectious-disease/clostridium-difficile-infection. Accessed October 11, 2015.
4. Mergenhagen KA, Wojciechowski AL, Paladino JA. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics.2014;32(7):639-650.
5. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
6. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-431.
7. Bakken JS, Borody T, Brandt LJ, et al. Treatment of Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044-1049.
8. Rohlke F, Stollman N. Fecal microbiota transplantation in relapsing Clostridium difficile infection. Ther Adv Gastroenterol. 2012;5(6):403-420.
9. Schwan A, Sjolin S, Trottestam U, et al. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet. 1983;2:845.
10. Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol. 2013;29:79-84.
11. Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079-1087.
12. Kelly CR, Kahn S, Kashyap P, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology. 2015;149:223-237.
13. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
14. Bakken JS. Feces transplantation for recurrent Clostridium difficile infection: US experience and recommendations. Microb Ecol Health Dis. 2015;26:27657. eCollection 2015.
15. Stollman N, Smith M, Giovanelli A, et al. Frozen encapsulated stool in recurrent Clostridium difficile: exploring the role of pills in the treatment hierarchy of fecal microbiota transplant nonresponders. Am J Gastroenterol. 2015;110:600-601.
16. Hirsch BE, Saraiya N, Poeth K, et al. Effectiveness of fecal-derived microbiota transfer using orally administered capsules for recurrent Clostridium difficile infection. BMC Infect Dis. 2015;15:91.
17. Van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;365(5):407-415.
18. Smits LP, Bouter KE, De Vos WM, et al. Therapeutic potential of fecal microbiota transplantation. Gastroenterol. 2013;145:946-953.
19. Cammarota G, Ianiro G, Gasbarinni A. Fecal microbiology transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48(8):693-702.
20. Brandt LJ, Reddy SS. Fecal microbiota transplantation in recurrent Clostridium difficile infection. J Clin Gastroenterol. 2011:45:S159-S167.
To comment on this article, contact rdavidson@uspharmacist.com.






