US Pharm. 2016;41(5):46-47.
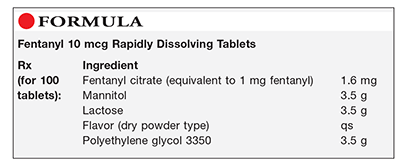
Method of Preparation: Note—The quantities of the excipients may be adjusted depending on the mold being used. Preblended rapidly dissolving tablet powder mixtures are available and may be used in place of the excipients in this preparation.
Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. Blend the fentanyl citrate, mannitol, lactose, and dry flavor until fine and uniformly mixed. Separately, reduce the particle size of the polyethylene glycol 3350 to approximately 200 mesh. Lightly blend the polyethylene glycol 3350 into the previously blended powders. Place 100 mg of the powder into the cavities of a mold (some blister packs work well; otherwise, obtain a tablet triturate mold or a special mold for preparing these tablets). Place the mold containing the powder in an oven at 80°C to 90°C for approximately 15 to 20 minutes. The time may vary depending upon the mold, formulation, oven, and so on. Remove from the oven and place in a refrigerator for approximately 5 minutes. Remove from the refrigerator and let set at room temperature. Package and label.
Packaging: Package in tight, light-resistant containers.1
Labeling: Keep out of reach of children. Use only as directed.
Stability: A beyond-use date of up to 6 months is appropriate for this preparation.1
Use: Fentanyl citrate rapidly dissolving tablets have been used as an analgesic for moderate-to-severe pain in appropriate patients.
Quality Control: Quality-control assessment can include weight–overall average weight, weight–individual weight variation, dissolution, active-drug assay results, physical appearance (color, uniformity), and physical stability (discoloration, changes).2
Discussion: This low-dose fentanyl citrate rapidly dissolving tablet may be used in children, and the dosage can be easily adjusted by altering the quantity per tablet or by administering multiple tablets. These tablets may be taken without water and can be administered almost anywhere.
Fentanyl citrate (C22H28N2O.C6H8O7, MW 528.6) occurs as white granules or as a white, crystalline powder. Fentanyl citrate 157 mcg is approximately equal to 100 mcg of fentanyl. It is soluble 1 g in 40 mL of water and is slightly soluble in alcohol. The pH of the fentanyl injection is in the range of pH 4.0 to 7.5. Fentanyl citrate is a synthetic opioid analgesic used as a sedative, analgesic, preoperative medication, and adjunct to general or regional anesthesia and in the management of chronic pain.3
Mannitol (C6H14O6, MW 182.17) occurs as a white, crystalline powder or as free-flowing granules. It is odorless and has a sweet taste. It is freely soluble in water, soluble in alkaline solutions, and very slightly soluble in alcohol. Mannitol is used as a sweetening agent, tablet and/or capsule diluent, tonicity-adjusting agent, and bulking agent for freeze-drying. It is preserved in well-closed containers. Mannitol has a melting range of 164°C to 169°C.1
Lactose (milk sugar, saccharum lactis, C12H22O11) is available either in anhydrous form or as the monohydrate. Commercially, lactose is available as anhydrous alpha-lactose, alpha-lactose monohydrate, and anhydrous beta-lactose, and it is available in different grades and particle characteristics. Lactose is widely used as a diluent in numerous dosage forms and in lyophilized products and infant formulas. It is soluble in water to the extent of about 1 g in 4.63 mL, but is practically insoluble in ethanol. Lactose exhibits a Maillard-type reaction (brownish discoloration) when it is heated with compounds containing a primary amine group; this reaction occurs more rapidly under alkaline conditions. Lactose is listed as being incompatible with amino acids, aminophylline, and amphetamines (owing to the amine groups present).4
Polyethylene glycol (Carbowax, PEG, polyoxyethylene glycol) is an addition polymer of ethylene oxide and water. At room temperature, polyethylene glycols with molecular weights >1,000 are solid. Solid polyethylene glycols are white or off-white pastes or waxy flakes. The density of the solid polyethylene glycols is in the range of 1.15 to 1.21 g/mL. The melting range for polyethylene glycol 3350 is between 48°C and 54°C. Polyethylene glycol is soluble in water, acetone, dichloromethane, ethanol, and methanol and slightly soluble in aliphatic hydrocarbons and ether.5
REFERENCES
1. United States Pharmacopeia—National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; April 2016.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of powder-filled, hard-gelatin capsules. IJPC. 1999;3:232-233.
3. Reynolds JE, ed. Martindale: The Extra Pharmacopoeia. 30th ed. London, England: Pharmaceutical Press; 1993:1076-1077.
4. Edge S, Kibbe AH, Shur J. Lactose, anhydrous. In: Rowe RC, Sheskey PJ, Cook WG, Fenton ME, eds. Handbook of Pharmaceutical Excipients. 7th ed. London, England: Pharmaceutical Press; 2012:410-412.
5. Price JC. Polyethylene glycol. In: Rowe RC, Sheskey PJ, Cook WG, Fenton ME, eds. Handbook of Pharmaceutical Excipients. 7th ed. London, England: Pharmaceutical Press; 2012:585-591.
To comment on this article, contact rdavidson@uspharmacist.com.





