US Pharm. 2007;32(3):34-38.
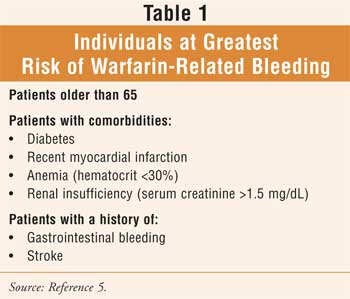
Blood clotting is a very complicated process. Approximately two million Americans require warfarin to prevent blood clotting after a stroke, heart attack, fracture, or surgery, or for long-term therapy in the treatment of atrial fibrillation.1 The appropriate dose of warfarin can vary greatly from patient to patient and is often difficult to predict.1-3 If the dose is too low, blood clots may develop, and if the dose is too high, it can result in excessive bleeding. Bleeding is the most common complication of anticoagulation therapy with warfarin, contributing to medication-related emergency department visits, morbidity, mortality, and increased health care expenditures. Age greater than 65, comorbidities (see Table 1), and a history of gastrointestinal bleeding or stroke are associated with the greatest risk of bleeding.4 Patients with two or three risk factors have a much higher incidence of warfarin-associated bleeding, compared to those with none or one.4
The risk of medication-related
adverse events in the elderly increases exponentially as the number of
medications in the regimen increases--due, in part, to polypharmacy--reflecting
the presence of comorbidities and the increased risk of drug and disease
interactions. Additionally, patients can further increase the risk of
complications when they self-medicate with OTC medications (e.g., aspirin,
ibuprofen, naproxen) and alternative therapies (e.g., ginkgo biloba) that are
known to increase the effect of warfarin.5,6
Pharmacists are actively
involved in warfarin monitoring and continue to counsel older patients on how
to best avoid side effects and medication interactions. It is important to
warn seniors and their caregivers that the elderly may respond differently to
alternative therapies than do younger individuals, especially since more than
50% of older people who use alternative therapies do not report the use of
such therapies to their physician.7 In general, lower warfarin
loading (<7.5 mg) and maintenance (usually <5 mg/day) doses are recommended
for elderly patients.8 It is important to recognize that in all
cases, it is a manipulation of the balance of risks.9 When
warfarin is temporarily stopped (e.g., prior to a surgical procedure), elderly
patients may achieve reversal to normal clotting function more slowly than
younger patients.8
Most hospitals provide a
warfarin clinic, which delivers outpatient services for dosing warfarin using
the standard "trial and error" basis. Clinicians use information primarily
about a patient's sex, age, weight, and medical history to establish the
initial warfarin dose. While several computer programs have been developed to
guide warfarin dosing, patients usually experience several months
of clinic appointments and blood sampling to determine an ideal dose; ongoing
dosage adjustments are based on close monitoring. Adherence to dietary and
lifestyle restrictions, as well as avoidance of medication (prescription and
OTC) interactions, is also required in an attempt to avoid fluctuations in the
international normalized ratio (INR).
While age does not alter the
pharmacokinetics of warfarin, it may have an effect on warfarin response in
seniors by increasing the prothrombin time or INR.8 This may occur
as a result of an elderly individual's increased sensitivity to the
anticoagulant effect of warfarin.5 Additionally, other factors
(e.g., genetic, environmental, developmental) may result in variations in
medication response among patients.5
The observed variability in patient response
to a particular medication may be due to genetically determined differences in
drug distribution, drug target proteins, and drug metabolism.10
Developing a genetically based dosing protocol for these patients could
prevent dangerous complications that result in tens of thousands of
hospitalizations and deaths each year. Through the use of technology to
determine genetic information, future personalizing or tailoring warfarin
dosing is predicted to save an estimated 1.1 billion in health care dollars in
the United States each year.11
Tests are already commercially available for
several enzymes whose variations result in different drug responses, including
two members of the cytochrome P450 family--CYP2D6 and CYP2D9.12
Additionally, the FDA has started to include pharmacogenetic considerations
in the prescribing information for some drugs.12
Pharmacogenetics and
Warfarin
The term
pharmacogenetics refers to variations in medication response secondary to
genetic makeup. A pharmacogenetic variation, causing reduced warfarin
activity, may be due to a genetically controlled reduction in binding affinity
of the warfarin receptor.5 Furthermore, about 10% of the difference
in people's responses to warfarin is attributed to variations in a gene
encoding the CYP2C9 enzyme, which metabolizes warfarin; tests for these
genetic variations, however, are not routinely performed.2 The
recently identified gene vitamin K epoxide reductase, or VKORC1, is a
key component in the clotting process and the primary target of warfarin.
13
A retrospective study funded
by the National Institute of Environmental Health Sciences, the National
Heart, Lung, and Blood Institute, and the National Institute of General
Medical Sciences revealed that genetic variations may influence a patient's
response to warfarin, according to findings published in the New England
Journal of Medicine.1,3,13 Patients from clinics at the
University of Washington in Seattle and Washington University in St. Louis
participated in the study. Researchers analyzed the genetic information from
patients taking warfarin and found that they fell into three dosage groups:
low, intermediate, and high.1,14 Genetic variations correlated with
the dosage groups, suggesting that the findings hold promise for streamlining
warfarin therapy.1,14 According to the lead researcher, the
VKORC1 gene appears to be the largest genetic variation affecting
warfarin, and it accounted for 25% of the overall variation in warfarin doses
in these studies.1,14 This study is one of the first to show a
major effect of genetics on the varying levels of medications that patients
require.1,13 The results are promising for simplifying warfarin
therapy by strategizing a more precise dosage range of warfarin that is needed
at the initiation of therapy and for eliminating many follow-up tests.1,14
The study team also discovered
that specific population groups tended to have a higher prevalence of a
particular VKORC1 variation.1,12 Asian Americans generally
had the low-dose variation or genotype, African Americans had the high-dose
genotype, and European Americans fell somewhere in the middle.1,3,13
The scientists recommended
that, because the studies were conducted among patients who already were
taking stable doses of warfarin, further studies are necessary in which the
choice of the initial dose of warfarin is based on identification of the
genetic variations.1,14
Prospective Clinical
Studies
The impetus for
prospective studies in a clinical setting has been noted. We will soon have
further infor mation to help determine whether knowledge of genetic variation
impacts positively on patient treatment with warfarin. Five teaching hospitals
affiliated with Harvard Medical School have begun a study to increase the
percentage of patients who receive the optimal warfarin dose.11 In
Wisconsin, the Marshield Clinic Research Foundation will conduct a new study
that will compare results between patients receiving warfarin via standard
"trial and error" dosing and those who receive genetic-based dosing.
11 A joint effort between prescription benefit manager Medco and the
Mayo Clinic's Center for Individualized Medicine will entail sending results
of genetic testing of patients with atrial fibrillation directly to their
respective physicians to ultimately facilitate the adoption of a more tailored
dosing strategy.

Summary
Clinicians continue to find warfarin
challenging to prescribe, despite its widespread use. Through the use of
technology to determine genetic information, future personalizing or tailoring
of warfarin dosing is estimated to save over a billion health care dollars in
the U.S. each year. Researchers recommend that genetic analysis of VKORC1
be an essential component of future prospective studies focusing on
investigating the value of genotyping for warfarin therapy. Prospective,
clinical study is now underway at multiple study sites. As scientists
accumulate more evidence and a better understanding of the genes associated
with varied medication responses, additional testing methods for relevant gene
variants will be developed. Pharmacogenetics will eventually shift from the
laboratory to clinical practice, with the goal of tailored dosing based on a
patient's individual characteristics.
References
1. Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285-2293.
2. Genetic Variation Alters Response to Common Anti-Clotting Drug. National Institute of General Medical Sciences, National Institutes of Health, Department of Health and Human Services. Available at: www.nigms.nih.gov/News/Results/060205.htm. Accessed January 29, 2006.
3. Rettie AE, Thummel KE. VKORC1 Gene Variants Control Response to the Common Anti-Clotting Drug Warfarin. National Institute of Environmental Health Sciences, National Institutes of Health. Available at: www.niehs.nih.gov/dert/profiles/hilites/2005/anticlot.htm. Accessed January 29, 2007.
4. Hirsh J, Fuster V, Ansell J, et al. American Heart Association/American College of Cardiology Foundation Guide to Warfarin Therapy. AHA/ACC Scientific Statement. Circulation. Available at: www.circ.ahajournals.org/cgi/content/full/107/12/1692. Accessed February 19, 2007.
5. Beers MH, Porter RS, Jones TV, et al. The Merck Manual of Diagnosis and Therapy. 18th ed. Whitehouse Station, NJ: Merck Research Laboratories; 2006:420, 759-760, 2514-2520, 2533-2545.
6. Semla TP, Beizer JL, Higbee MD. Geriatric Dosage Handbook. 10th ed. Cleveland: Lexi-Comp, Inc.; 2005.
7. Beers MH, Jones TV, Berkwits M, et al. The Merck Manual of Health & Aging. Whitehouse Station, NJ: Merck Research Laboratories; 2004:54-55.
8. Beers MH, Berkow R. The Merck Manual of Geriatrics. 3rd ed. Whitehouse Station, NJ: Merck & Co.; 2000:60-61, 70-71.
9. Matulis M, Knovich M, Owen J. Thrombotic and Hemorrhagic Disorders. In: Hazzard WR, Blass JP, Halter JB, et al. Principles of Geriatric Medicine and Gerontology. 5th ed. New York: McGraw-Hill, Inc.; 2003:803-817.
10. Phillips KA, Veenstra DL, Oren E, et al. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA. 2001;286:2270-2279.
11. Johnson LA. DNA Tests to Determine Warfarin Dose. The Associated Press; January 13, 2007. Available at: www.washingtonpost.com/wpdyn/content/article/2007/01/13/AR2007011300264.html. Accessed February 9, 2007.
12. Pharmacogenetics Research Leads the Way to Individualized Therapy. National Institute of General Medical Sciences, National Institutes of Health, Department of Health and Human Services. Available at: www.nigms.nih.gov/News/Briefs/PharmacogeneticsResearch.htm. Accessed January 29, 2007.
13. Determining drug dose with DNA: Graduate School alumnus leads study of genetic influence on response to common blood thinner. Available at: www.mcw.edu/display/router.asp?docid=15475. Accessed January 29, 2007.
14. Altman LK. Study Suggests Gene
Tests Could Ease Use of Anti-Clotting Drug. New York Times. June 2,
2005. Available at:
www.nytimes.com/2005/06/02/health/02blood.html?ex=1275364800&en=ca725aad373c64b5&ei=5088&partner=rssnyt&emc=rss.
Accessed January 29, 2007.
To comment on this article,
contact editor@uspharmacist.com.





