US Pharm. 2015;40(9):HS15-HS20.
ABSTRACT: Proton pump inhibitors (PPIs) and histamine2 receptor antagonists (H2RAs) are heavily utilized gastric acid suppressants, and more potential adverse effects are being attributed to these agents. With gastrointestinal conditions increasingly commonplace, many patients are using PPIs continuously for years even though most of the drugs lack extensive long-term data and are FDA-approved for much shorter durations of therapy. An area of controversy, but with a considerable and still-growing body of evidence, is the association of PPIs and perhaps H2RAs with fractures, particularly in postmenopausal women.
As a class, gastric acid suppressants, such as proton pump inhibitors (PPIs) and histamine2 receptor antagonists (H2RAs), have become a mainstay in both OTC and prescription treatment for various gastrointestinal (GI) conditions, including duodenal ulcer disease, erosive esophagitis, gastroesophageal reflux disease (GERD), heartburn, Helicobacter pylori eradication, and hypersecretory conditions (e.g., Zollinger-Ellison syndrome).1,2 PPIs are most commonly indicated for a 4- to 8-week duration (sometimes up to 12-16 weeks) (TABLE 1).1,2 An additional period of maintenance therapy may be indicated, particularly in patients with hypersecretory disorders. The studies submitted to the FDA to acquire indications for approval for longer-term therapy were focused on demonstrating efficacy, but the sample sizes were too small to evaluate long-term safety.3-5
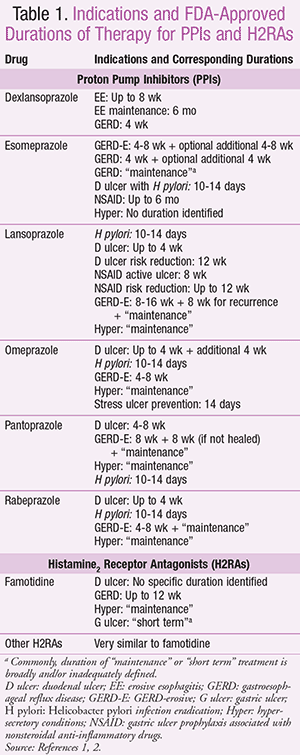
In its most recent guidelines (2013), the American College of Gastroenterology recommends an initial 8-week course of PPIs as the therapy of choice for symptom relief and healing of erosive esophagitis.6 Further, the guidelines recommend that maintenance PPI therapy be prescribed in patients with GERD complications, including erosive esophagitis or Barrett’s esophagus, and in those who continue to have symptoms after PPIs have been discontinued. They state that long-term PPI therapy should be prescribed at the lowest effective dose and that intermittent therapy can be considered. Alternatively, H2RA therapy may be effective for relief of continued heartburn symptoms in patients with uncomplicated disease.
Long-term PPI therapy is also strongly recommended in patients with nonsteroidal anti-inflammatory drug (NSAID)- or aspirin-induced ulcers in whom continued NSAID or aspirin therapy is required.7 The FDA-approved indication for prevention or treatment of NSAID-induced ulcers is generally for a duration of 8 to 12 weeks (TABLE 1),1,2 although maintenance therapy is often required.7
General Safety Concerns
Long-term use of PPIs can increase the risk for fractures, especially hip fractures. The FDA has revised the prescription and OTC labels for this drug class to include safety information about a possible increased risk of fractures of the hip, wrist, and spine.8 In fact, over the last few years the FDA has issued several Drug Safety Communications and revised the product labeling of PPIs in response to the emerging data.9 Other emerging safety concerns with the PPIs that should be considered by pharmacists include pneumonia, electrolyte/mineral deficiencies, Clostridium difficile infections, and increased risk of myocardial infarction (MI) in the general population.4,5,9-11
Despite the OTC and generic availability of several PPIs and H2RAs and the association of these drugs with various safety concerns, gastric acid suppressants still represent a top-10 drug expense for third-party insurers.12
Long-Term Safety of PPIs
There have been several long-term studies (some >1 year) to demonstrate PPI efficacy, particularly those used to obtain FDA approval for hypersecretory conditions.3-5 Rarely have these studies been large enough to detect serious adverse outcomes.
Several PPIs have indications for use for specific conditions for a relatively undefined period of maintenance therapy (TABLE 1).1,2 Sample size and duration of the supporting studies make assessment of safety difficult when considering maintenance therapy for a specific patient. This leaves us to analyze retrospective analyses, pooled analyses, subgroups in the larger studies, and pharmacosurveillance studies.
In the recent study describing PPI use and a statistically significant risk of MI in the general population, the authors specifically and repeatedly note that if pharmacovigilance studies had been in place to track long-term use with lansoprazole, then the MI risk with lansoprazole could have been flagged as early as the year 2000.11 Other pharmaco-surveillance studies have indicated a significant association between PPI use and increased mortality in persons aged >65 years (average age 80 years) with a hazard ratio (HR) of 1.51 (95% CI, 1.03-2.77). Higher doses of PPIs were particularly associated with mortality: HR of 2.59 (95% CI, 1.22-7.16).13
The underlying association between PPI use and mortality is likely complex, multifactorial, and not clearly correlated; many of the proposed long-term safety issues (including fractures) with PPIs could contribute.
Bone Fractures
Fractures, especially hip fractures, must be prevented whenever possible. Mortality and loss of functionality due to hip fractures in older adults is associated with significantly increased morbidity and mortality, especially for those in long-term care facilities, who have a mortality rate of 36% within 180 days of fracture.14
Until 2012, the strongest evidence for the association between long-term PPI use and fracture was a meta-analysis published in 2006 that demonstrated a risk of hip fracture in those who used PPIs for >1 year.15 As the dose or duration of PPI therapy increased, so did the risk for fracture. At 1 year, the odds ratio (OR) for hip fracture was significant and continued to increase steadily up to the 4-year mark. The investigators adjusted for many factors, which lowered adjusted OR values. This indicated that there are many underlying factors other than acid-lowering therapy that may contribute to the risk of fracture. In fact, the investigators were able to control for many of these factors, such as smoking status.
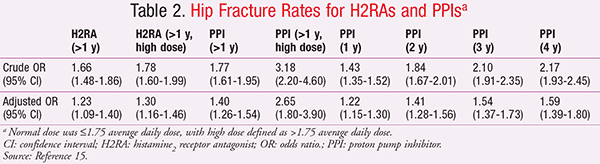
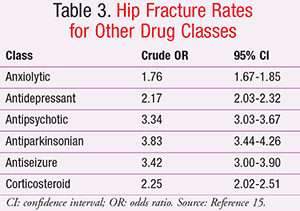
In this particular study, H2RAs were also associated with increased hip fractures, but less so than the PPIs (TABLE 2).15 It is helpful to review the crude hip fracture rates reported with other medication classes against which the acid-lowering therapies were compared in this study (TABLE 3).15 Antipsychotic, antiparkinsonian, and antiseizure drugs were all associated with hip fracture rates similar to or higher than those seen with high-dose PPI therapy >1 year. Many of the classes listed in TABLE 3 that were associated with hip fracture risk negatively impact bone mineral density (BMD) and include many agents considered potentially inappropriate in the elderly, often due to the risk for falls.15,16
A more recent meta-analysis of 11 observational studies looked at the use of acid-suppressant medications and the risk for fracture and found that there was an increased risk of both hip fractures (adjusted OR = 1.34; 95% CI, 1.09-1.66) and general fractures (adjusted OR = 1.30; 95% CI, 1.15-1.48) with “any use” as well as “long-term use” of PPIs. An increased risk of fracture was not detected with “any use” or with “long-term use” of H2RAs.17
The largest prospective study to date evaluated the use of PPIs and risk of hip fracture in relation to dietary and lifestyle factors.18 The participants included 79,899 postmenopausal women (over 565,784 person-years) in the Nurses’ Health Study in which 893 hip fractures were documented. Although this study did not evaluate PPI dose, it did evaluate duration of PPI use (up to 6-8 years) and the use of H2RAs. Both PPIs and H2RAs were associated with a significant increase risk of hip fracture with an HR of 1.36 (95% CI, 1.12-1.65) and 1.23 (1.02-1.50), respectively. Use of <2 years was not associated with fracture risk, and there was a significant trend associating longer use at 2, 4, and 6 to 8 years with increased hip fracture risk. These results held especially true in a multivariate analysis after controlling for many factors associated with bone health. Only one factor, smoking status, was found to dramatically and independently influence whether PPI use was associated with fracture. Risk for fractures in nonsmokers was not significantly increased with PPI use according to the multivariate adjusted HR of 1.06 (0.77-1.46), especially in comparison to smokers who bore a significantly increased risk: HR = 1.51 (1.20-1.91). It should also be noted that having discontinued PPI use for 2 years or more demonstrated a hip fracture risk similar to those who never used PPIs.18
The most recent guideline from the American College of Gastroenterology does not endorse early PPI withdrawal, even in patients with osteoporosis or concern for fractures.6
Are H2RAs Associated With Less Fracture Risk Than PPIs?
Not only are there limited high-quality safety data on long-term use of PPIs, quality comparator data are even more lacking. In the studies presented thus far, most, but not all, of the available data indicate an association between PPI use and fractures; only a few studies find this same association with H2RAs. Two studies that did and one that did not find this association were previously reviewed.15,17,18 A 2011 meta-analysis including studies conducted predominantly in postmenopausal women and men of similar age found an increased risk of fractures in participants using PPI therapy, but no association was found between H2RA therapy and increased risk of fractures.19
In a different type of analysis from the United Kingdom, investigators identified patients aged 40 to 89 years with a recorded history of hip fracture and compared them to controls with similar demographics from the Health Improvement Network database.20 They found the risk of hip fracture increased with PPIs (high doses were worse than lower doses) and with high doses of H2RAs. Medium-dose acid suppressant therapy included esomeprazole 40 mg, omeprazole 20 mg, lansoprazole 30 mg, pantoprazole 40 mg, rabeprazole 20 mg, cimetidine 800 mg, famotidine 40 mg, nizatidine 300 mg, and ranitidine 300 mg; doses higher than these were defined as “high.” The authors further identified that the current health and physical condition of the patient plays a significant role in the incidence of hip fracture with PPI or H2RA use. In fact, they concluded that baseline health status may have been the greatest contributing factor. An association between other pharmacologic treatments and increased risk of fracture supported this conclusion, as even bisphosphonates and vitamin D plus calcium supplements were associated with increased fracture risk in this particular analysis.20
Do PPIs Reduce Bone Mineral Density?
Because an increase in fracture risk has been reported, patients and prescribers may be concerned that acid suppression therapy may be increasing the risk for developing osteoporosis. Multiple longitudinal analyses have been conducted to examine the relationship between PPI use and changes in BMD or fracture risk.20-24 None of these studies found a significant association between use of PPIs and decreases in BMD. In one of these articles, the authors were unable to demonstrate loss of BMD caused by PPI use even after 10 years of follow-up with patients using PPIs.24
In the most recent study, Solomon et al25 corroborated these results by analyzing data available from the Study of Women’s Health Across the Nation (SWAN).26 To minimize confounders, they included only new users of acid-suppressant therapies and also conducted a positive control analysis with hormone replacement therapy. Their goal was to describe the relationship between use of a PPI, H2RA, or no acid-suppressant therapy and changes in BMD in women undergoing the menopause transition. These investigators found no link between PPI or H2RA use and loss of bone density in midlife women who initiated medication use during the study.
An Alternative Explanation May Be an Increase in Risk of Falls
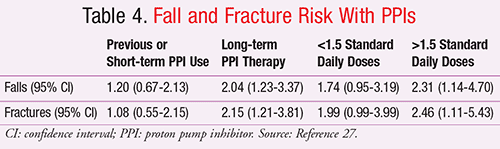
Lewis et al reported the results of two analyses looking at the association between long-term PPI use and risk of falls and fracture in elderly women.27 The first study was an a priori, post hoc analysis of data from the Calcium Intake Fracture Outcome Study (CAIFOS), and then a second prospective (replication) cohort study was undertaken to validate the results. In the retrospective cohort, the adjusted odds of experiencing an injurious fall were 2.04 (95% CI, 1.23-3.37) in long-term users of PPIs and the odds of fracture were 2.15 (95% CI, 1.21-3.81) (TABLE 4). The findings of the second study were limited to an adjusted 51% increase in self-reported falling (P = .049), worse self-reported falls-associated metrics (e.g., dizziness, numbness of the feet), and significantly reduced serum cyanocobalamin levels in the PPI group. Bone structure analyses as determined by total hip BMD, whole body BMD, and quantitative heel ultrasound did not differ between PPI exposure categories. The authors concluded that in this study and previous trials any association that may exist with PPI therapy and fracture is independent of bone structure.27
Conclusion and the Pharmacist’s Role
As pharmacist involvement in patient-centered care and team-based medicine continues to increase, pharmacists will be called upon to provide up-to-date information on this and related controversial topics. Pharmacists will also continue to provide consultation or medication therapy management for patients taking PPIs or H2RAs, as well as recommend OTC treatments for GI conditions. Pharmacists should discuss correct use and reasonable expectations with patients using acid-lowering therapy to encourage patients to utilize the lowest effective dose for the shortest duration necessary.
Although PPIs are generally considered safe and effective, evidence is building to corroborate concerns, in agreement with the FDA-required labeling, that these medications can cause an increased risk of fracture, and this may be due primarily to an increased risk for falls. Multiple studies suggest that PPI use is associated with increased risk of fracture, but not of BMD loss. Hip fractures, particularly for those in long-term care, are associated with significant morbidity and mortality. Rarely have the safety profiles of H2RAs and PPIs been compared head-to-head, so it is difficult to conclude whether H2RAs are truly associated with less fracture risk. High-dose PPIs do seem to be associated with the greatest risk, and stepping down therapy to the lowest effective dose is practical. PPIs should also be discontinued if there is no clear indication for their use, particularly in patients already at risk for falls and fractures. Pharmacists have a major role in helping patients find cost-effective ways to treat GI disorders safely and effectively, in identifying potentially unnecessary drug therapy, and in reducing the risk of falls and fractures in postmenopausal women and into the later years of life.
REFERENCES
1. Package inserts for dexlansoprazole, esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole, famotidine, and ranitidine. Lexi-Comp [mobile application database]. Accessed June 16, 2014.
2. Package inserts for dexlansoprazole, esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole, famotidine, and ranitidine. Micromedex [online database]. www.micromedexsolutions.com. Accessed June 11, 2015.
3. Metz DC, Sostek MB, Ruszniewski P. Effects of esomeprazole on acid output in patients with Zollinger-Ellison syndrome or idiopathic gastric acid hypersecretion. Am J Gastroenterol. 2007;102:2648-2654.
4. Nexium (esomeprazole magnesium) prescribing information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; March 2014.
5. Prevacid (lansoprazole) prescribing information. Deerfield, IL: Takeda Pharmaceuticals America, Inc; September 2012.
6. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328.
7. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345-360.
8. FDA Drug Safety Communication. Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. May 25, 2010. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed June 11, 2015.
9. FDA Drug Safety and Availability. Information by class: proton pump inhibitors information. February 9, 2012. www.fda.gov/drugs/drugsafety/informationbydrugclass/ucm213259.htm. Accessed June 11, 2015.
10. Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 2013;6(4): 443-451.
11. Shah NH, LePendu P, Bauer-Mehren AB. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One. 2015;10(6):e0124653.
12. Express Scripts Drug Trend Report, 2014. http://lab.express-scripts.com/drug-trend-report/. Accessed June 11, 2015.
13. Maggio M, Corsonello A, Ceda GP. Proton pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173(7):518-523.
14. Neuman MD, Silber JH, Magaziner JS, et al. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174;1273-1280.
15. Yang Y, Lewis JD, Epstein S. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-2953.
16. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
17. Eom C, Park SM, Myung S. Use of acid-suppressive drugs and risk of fracture: a meta-analysis of observational studies. Ann Fam Med. 2011;9: 257-267.
18. Khalili H, Huang ES, Jacobson BC, et al. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012;344:e372.
19. Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124(6): 519-526.
20. Cea Soriano L, Ruigómez A, Johansson S, García Rodríguez LA. Study of the association between hip fracture and acid-suppressive drug use in a UK primary care setting. Pharmacotherapy. 2014;34(6):570-581.
21. Yu EW, Blackwell T, Ensrud E, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int. 2008;83(4): 251-259.
22. Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138:896-904.
23. Targownik LE, Leslie WD, Davison KS, et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study [corrected] from the Canadian Multicentre Osteoporosis Study (CaMos). Am J Gastroenterol. 2012;107(9):1361-1369.
24. Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture and change in bone density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med. 2010;170(9):765-771.
25. Solomon DH, Diem SJ, Ruppert K, et al. Bone mineral density changes among women initiating proton pump inhibitors or H2 receptor antagonists: a SWAN Cohort study. J Bone Min Res. 2015;30(2):232-239.
26. Corletoa VD, Festaa S, Giulio ED, Annibale B. Proton pump inhibitor therapy and potential long-term harm. Curr Opin Endocrinol Diabetes Obes. 2014;21:3-8.
27. Lewis JR, Barre D, Zhu K, et al. Long-term proton pump inhibitor therapy and falls and fracture in elderly women. J Bone Miner Res. 2014;29(11):2489-2497.
To comment on this article, contact rdavidson@uspharmacist.com.





