US Pharm. 2009;34(8):70-71.
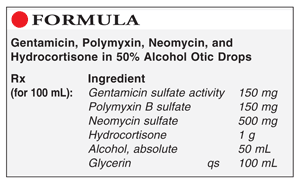
Method of Preparation: Calculate the required quantity of each ingredient for the total amount to be prepared. The quantity of gentamicin sulfate must be calculated from the activity labeled on the bulk container or the certificate of analysis for that specific lot. Accurately weigh or measure each ingredient. Combine the powders. Add about 10 mL of glycerin and mix well. Incorporate the alcohol and mix well. Add sufficient glycerin to volume and mix well. Package and label.
Use: This compound has been used for the treatment of swimmer's ear.
Packaging: Package in a tight, light-resistant dropper container.
Labeling: For the ear. Use only as directed. Keep out of the reach of children.
Stability: A beyond-use date of 6 months can be used for this preparation.1
Quality Control: Quality-control assessment can include weight/volume, specific gravity, active drug assay, color, clarity, rheologic properties, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth).2
Discussion: Summer is a busy time at the swimming hole, with resultant earaches and ear infections. Many different options are available depending upon the severity of the patient's discomfort, even extending to ear infections. A vehicle containing alcohol, such as the one presented here, will assist in breaking the surface tension of any entrapped water and enable deeper penetration of the vehicle containing the antibiotics.
Gentamicin sulfate is an aminoglycoside antibiotic that occurs as the sulfate salt, or a mixture of such salts, of the antibiotic substances produced by the growth of Micromonospora purpurea. It occurs as a white- to buff-colored powder that is soluble in water, but insoluble in alcohol. It has a potency equivalent of not less than 590 mcg of gentamicin per mg calculated on the dried basis. Gentamicin sulfate should be preserved in a tight container.3
Polymyxin B sulfate is the sulfate salt of a polymyxin produced by the growth of Bacillus polymyxa. It occurs as a white- to buff-colored hygroscopic powder that is odorless or has a faint odor. Polymyxin B sulfate is freely soluble in water and in 0.9% sodium chloride injection; it is slightly soluble in alcohol. It has a potency of not less than 6,000 polymyxin B units per mg, calculated on the dried basis. Each mg of pure polymyxin B is equivalent to 10,000 units of polymyxin B activity. Its aqueous solutions have a pH of 5 to 7.5.1,3
Neomycin sulfate is an aminoglycoside antibiotic obtained from cultures of Streptomyces fradiae. It occurs as a white to slightly yellow hygroscopic powder that is freely soluble in water and very slightly soluble in alcohol. It should be stored in a tight, light-resistant container. Neomycin sulfate has a potency equivalent of not less than 600 mcg of neomycin per mg, calculated on the dried basis.1,3
Hydrocortisone (C21H30O5, MW 362.46, cortisol, compound F) is a corticosteroid secreted by the adrenal cortex. It occurs as a white to practically white, odorless, crystalline powder. It is very slightly soluble in water and sparingly soluble in alcohol. Generally, either hydrocortisone or hydrocortisone acetate is applied topically to the eye or the ear.3
Alcohol (C2H5OH, MW 46.07, ethyl alcohol, ethanol, grain alcohol) is a clear, colorless, mobile, and volatile liquid with a slight characteristic odor and a burning taste. Alcohol USP refers to 95% ethanol; dehydrated or absolute alcohol refers to 99.5% alcohol. Its specific gravity is between 0.812 and 0.816, and its boiling point is 78.15°C. It is miscible with glycerin and water, and its solutions may be sterilized by autoclaving or filtration. Alcohol should be stored in a cool place. It is incompatible with oxidizing materials in acidic conditions. With alkalies, alcohol may darken in color. When it is added to aqueous solutions of organic salts or acacia, they may precipitate. Alcohol is incompatible with aluminum containers, and it may interact with some drugs.4
Glycerin (glycerol, 1,2,3-propane triol) occurs as a clear, colorless, odorless, viscous, hygroscopic liquid with a sweet taste about two-thirds as sweet as sucrose. It is miscible with water, methanol, and 95% ethanol; it is practically insoluble in oils and chloroform, and slightly soluble in acetone. Glycerin is hygroscopic and should be stored in an airtight container in a cool place.5
REFERENCES
1. USP Pharmacopeial Convention, Inc. USP Pharmacists' Pharmacopeia. 2nd ed. Rockville, MD: US Pharmacopeial Convention, Inc; 2005:189,197-198,255,288,764-
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
3. McEvoy GK. AHFS Drug Information 2008. Bethesda, MD: American Society of Health-System Pharmacists; 2008:75-81,83-84,493-495,3580-
4. Owen SC. Alcohol. In: Rowe RC, Sheskey PJ, Owen SC, eds. Handbook of Pharmaceutical Excipients. 5th ed. Washington, DC: American Pharmaceutical Association; 2006:18-20.
5. Price JC. Glycerin. In: Rowe RC, Sheskey PJ, Owen SC, eds. Handbook of Pharmaceutical Excipients. 5th ed. Washington, DC: American Pharmaceutical Association; 2006:301-303.





