US Pharm. 2016;41(8):41-44.
ABSTRACT: Gonorrhea is the second most common communicable disease in the United States. From 2010 to 2014, the rate of this sexually transmitted disease increased by 10.5% in the U.S. Cefixime, which was once the first-line agent for the treatment of Neisseria gonorrhoeae, is rapidly waning in efficacy, as are several other cephalosporins. Similarly, fluoroquinolone-resistant strains of N gonorrhoeae have been reported. As a result, the use of these agents has decreased. The CDC currently recommends dual treatment with ceftriaxone and azithromycin for most gonococcal infections. Multidrug-resistant gonorrhea is becoming a serious health threat in the U.S.
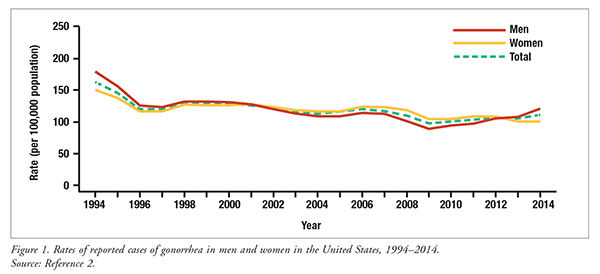
Neisseria gonorrhoeae is the second most common communicable disease in the United States, behind Chlamydia trachomatis.1 The CDC estimates that >800,000 new cases of N gonorrhoeae infection occur every year in the United States.1 From 2010 to 2014, the national rate of reported N gonorrhoeae infection increased 10.5%, from 100.2 cases to 110.7 cases per 100,000 people (FIGURE 1).2 This increase is primarily due to an upsurge in the number of males who contracted N gonorrhoeae.2
Classification
N gonorrhoeae infections may be classified as either uncomplicated or complicated. Uncomplicated infections are much more common, encompassing any urogenital, anogenital, or pharyngeal infection caused by N gonorrhoeae that does not result in bacteremia. An N gonorrhoeae infection that results in bacteremia and/or the spread of bacteria to joints and tissues is considered a complicated infection.3,4
Risk Factors
Risk factors for N gonorrhoeae include sexual contact with new or multiple partners, sexual contact with an individual who has concurrent partners, and sexual contact with a person who is currently infected with N gonorrhoeae. Other risk factors include inconsistent condom use while engaging in sexual contact with nonmonogamous partners; previous history of N gonorrhoeae infection; and trading sex for money, drugs, or other items.1
To help prevent the spread of infection, the CDC recommends that all sexually active women aged <25 years, as well as older women with multiple risk factors, be screened for N gonorrhoeae annually. Men who have had sexual contact with other males within the preceding year should be screened at least annually at the site of possible exposure (i.e., urethral, rectal, or pharyngeal). Men aged <35 years and women aged <30 years who reside in correctional facilities should be screened for N gonorrhoeae at the time of intake, regardless of risk factors. Pregnant women aged <25 years should be screened at the first prenatal visit.1
Clinical Signs and Symptoms
Many genital gonococcal infections are asymptomatic; however, these infections are more likely to be symptomatic in men than in women.5 Male urogenital symptoms include signs of urethritis or epididymitis, such as dysuria or unilateral testicular swelling.5 Males with extragenital infections of the rectum are often asymptomatic, but patients may present with signs of proctitis, such as constipation, rectal pain, and rectal bleeding.5 Those with pharyngeal gonococcal infections are usually asymptomatic, but if symptoms are present, they may include sore throat and pharyngeal exudates.5
Symptoms of N gonorrhoeae often prompt men to seek medical attention prior to the development of complications, but not soon enough to prevent transmission to other people. Most women remain asymptomatic until the development of complications such as pelvic inflammatory disease.1
Several sexually transmitted and non–sexually transmitted pathogens, as well as certain noninfectious processes, may present with signs and symptoms that are similar to those occurring in N gonorrhoeae infection. Therefore, a preliminary diagnosis based on history and physical examination should be confirmed through microbiologic testing.5 Nucleic acid amplification testing (NAAT) is typically used for initial microbiologic confirmation of N gonorrhoeae; in addition, cultures may be required if antibiotic resistance is a concern. For males, a urethral swab is sufficient for NAAT, but females should receive a vaginal or endometrial swab. Regardless of symptoms, the NAAT method should be implemented prior to a confirmed diagnosis of N gonorrhoeae infection.5 Suspected N gonorrhoeae infections are often treated empirically before completion of NAAT.4
Treatment
N gonorrhoeae is well known for its ability to adapt to and resist microbial therapies. Updated guidelines were published in 2015, including new treatment regimens for multidrug-resistant N gonorrhoeae.1 See TABLE 1 for a summary of treatment regimens for gonococcal infections. The new recommendation for the treatment of uncomplicated gonococcal infections of the cervix, urethra, and rectum is for a single dose of ceftriaxone 250 mg given IM, in addition to a single dose of azithromycin 1 g given orally. These medications should be administered in the healthcare provider’s clinic under direct supervision, if possible, to ensure that the patient completes therapy.1 Azithromycin is preferred over a tetracycline because it has a lower rate of N gonorrhoeae resistance.
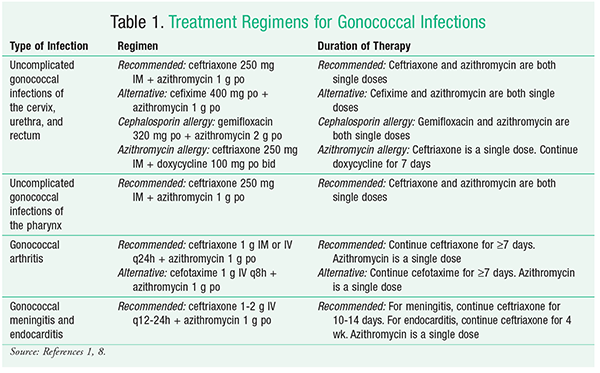
Another key consideration in therapeutic decision making is patient adherence, which is more likely with a one-time oral dose of azithromycin than with alternative multidose therapy. Moreover, azithromycin is an effective therapeutic option for uncomplicated genital C trachomatis infections. Therefore, this dual-therapy regimen is an appealing option because of the possibility of gonococcal and chlamydial coinfection.1 Doxycycline may be used as an alternative if the patient is allergic to azithromycin. If doxycycline is used, it should be dosed at 100 mg orally twice daily for 7 days.1 In male patients, doxycycline may also be used to treat epididymitis or proctitis caused by gonococcal infections.4
If ceftriaxone is not available at the time of treatment, cefixime may be used as an alternative. However, N gonorrhoeae has developed increased resistance to cefixime. For this reason, cefixime should be used only when ceftriaxone is not available.1,2 When used, cefixime should be given as a single oral dose of 400 mg.1 If the patient has a cephalosporin allergy, dual treatment with single oral doses of gemifloxacin 320 mg and azithromycin 2 g is an option. Alternatively, gentamicin may be given as a single dose of 240 mg IM in place of the gemifloxacin.1
Uncomplicated N gonorrhoeae infections of the pharynx are much more challenging to eradicate than uncomplicated urogenital and anorectal infections, and reliable cure rates of >90% of infections are achieved by very few antibiotic regimens.1 The CDC currently recommends that patients with uncomplicated gonococcal infections of the pharynx be treated with a regimen consisting of a single dose of ceftriaxone 250 mg IM in addition to a single oral dose of azithromycin 1 g. Clinical trials showed that the treatment of pharyngeal infections with ceftriaxone resulted in a cure rate of 98.9%.1
Patients with uncomplicated gonococcal infections of the cervix, urethra, and/or rectum do not require a follow-up test of cure if they are treated with a recommended first-line or alternative regimen.1 Patients with uncomplicated N gonorrhoeae pharyngeal infections do not require a test of cure if they are treated with ceftriaxone and azithromycin; however, if an alternative regimen is used, the patient should return to the physician’s office 14 days after therapy to receive a test of cure.1
Complicated gonococcal infections are much rarer than uncomplicated infections and may lead to serious conditions, such as septic arthritis, endocarditis, and/or meningitis. Patients with complicated gonococcal infections resulting in arthritis should receive ceftriaxone 1 g IM or IV every 24 hours for a minimum of 7 days, in addition to a single oral azithromycin dose of 1 g. If ceftriaxone is unavailable, cefotaxime 1 g every 8 hours may be administered IV for a minimum of 7 days and accompanied by a single oral azithromycin dose of 1 g.1,6 CDC guidelines state that patients with arthritis-dermatitis syndrome may be stepped down to oral therapy guided by antimicrobial susceptibilities once clinical improvement has been noted for 24 to 48 hours, for a total therapy duration of at least 7 days.1 Patients who develop gonorrheal meningitis receive ceftriaxone 1 to 2 g IV every 12 to 24 hours for 10 to 14 days, plus a single oral dose of azithromycin 1 g. Patients who develop gonorrheal endocarditis should receive the same agents, but ceftriaxone should be administered for at least 4 weeks.1
Antibiotic Resistance
When treating gonococcal infections, providers should be aware of possible antibiotic resistance. Researchers are concerned about the emergence of N gonorrhoeae as a superbug and its potential future resistance to all classes of antibiotics.7 Penicillin was once widely used to treat N gonorrhoeae, resulting in a mutation that allowed N gonorrhoeae to produce beta-lactamase. This enzyme is responsible for breaking down the beta-lactam ring of penicillin and rendering the bacteria nonsusceptible to penicillin’s therapeutic effects.8 Quinolones were also previously employed as a gonococcal treatment option. In 2005, quinolone-resistant N gonorrhoeae was reported at 89% of locations of the Gonococcal Isolate Surveillance Project (a service that monitors and reports trends of N gonorrhoeae infections in the U.S.). Because of this resistance, quinolones are no longer recommended for the first-line treatment of any type of gonococcal infection.3 Azithromycin monotherapy given as a single oral dose of 2 g has demonstrated effectiveness against uncomplicated urogenital gonorrhea; however, it is not recommended because N gonorrhoeae has the ability to easily develop resistance to macrolides and because this regimen has the potential for increased gastrointestinal side effects.1,3,9 Although cephalosporins remain the drug class most effective for treating gonococcal infections, some reductions in susceptibility have been documented.3,9
Since the 1930s, gonorrhea has been treated with, and developed resistance to, sulfonamides, penicillin, tetracycline, spectinomycin, quinolones, macrolides, and some cephalosporins.7 Antimicrobial stewardship, prescriber awareness, and appropriate patient education can help prevent N gonorrhoeae from developing resistance to ceftriaxone.
In 2012, the World Health Organization developed a global action plan to reduce antibiotic resistance to N gonorrhoeae.10 The plan includes encouraging early detection and effective treatment, fostering patient compliance, educating patients, improving surveillance and laboratory capacities, increasing advocacy, and ensuring that appropriate legislative and regulatory mechanisms are in place.10 Fostering patient compliance is especially important when multidose treatment regimens are employed.
Prevention and Management Considerations
Appropriate treatment is of paramount importance for existing gonococcal infections, but preventive measures must also be considered and discussed with the patient. Obtaining an accurate sexual history from the patient is vital. Although it is sometimes uncomfortable to do so, the healthcare provider and the patient must have a frank discussion about the patient’s sexual behavior.11 The CDC emphasizes the use of a sexually transmitted infection (STI) and HIV risk assessment in counseling high-risk patients. This may be accomplished by use of the Five P’s, which are detailed, open-ended questions designed to elicit more information about a patient’s sexual partners, sexual practices, pregnancy prevention, STI protection, and past history of STIs.1 The Five P’s method fosters an open conversation between the healthcare provider and patient for a better understanding of the patient’s risk profile. After risk assessment, individualized counseling based on responses may be undertaken.
Because males with a gonococcal infection often are asymptomatic, they may remain undiagnosed for an extended period of time, which makes prevention a key priority in high-risk male populations. Consistent use of male condoms should be recommended to all patients.1
The partner of a patient with a gonococcal infection should be referred for evaluation and probable treatment if he or she had sexual contact with the patient within 60 days prior to diagnosis or onset of symptoms, or if he or she was the patient’s last sexual partner. Unprotected sexual intercourse should be avoided until 7 days after treatment of both partners, and for as long as either partner is symptomatic.1
Conclusion
Most new N gonorrhoeae infections in the U.S. are uncomplicated and involve the urogenital, anogenital, or pharyngeal area. Complicated infections resulting in bacteremia, septic arthritis, endocarditis, or meningitis are less common. Individuals who engage in unsafe sexual practices and those with a history of past gonococcal infections are at increased risk for contracting N gonorrhoeae. Coinfection with C trachomatis must be considered in the selection of the most appropriate treatment. Ceftriaxone and azithromycin are the recommended first-line regimen for most N gonorrhoeae infections. To reduce repeat infections and the growth of potential resistance to dual therapy, the prevention measures discussed in this article must be emphasized in both preexposure and postexposure patient populations. Early detection, appropriate antibiotic selection, strict adherence to multidose treatment regimens, and communication with the patient are crucial to the effective management of gonorrhea.
REFERENCES
1. Workowski KA, Bolan GA; CDC. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
2. CDC. Sexually Transmitted Disease Surveillance 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2015.
3. Newman LM, Moran JS, Workowski KA. Update on the management of gonorrhea in adults in the United States. Clin Infect Dis. 2007;44(suppl 3):S84-S101.
4. Swygard H, Seña AC, Cohen MS. Treatment of uncomplicated gonococcal infections. UpToDate. www.uptodate.com/contents/treatment-of-uncomplicated-gonococcal-infections. Accessed February 12, 2016.
5. Ghanem KG. Clinical manifestations and diagnosis of Neisseria gonorrhoeae infection in adults and adolescents. UpToDate. www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-neisseria-gonorrhoeae-infection-in-adults-and-adolescents. Accessed February 12, 2016.
6. Goldenberg DL, Sexton DJ. Disseminated gonococcal infection. UpToDate. www.uptodate.com/contents/disseminated-gonococcal-infection. Accessed February 17, 2016.
7. Unemo M, Nicholas RA. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol. 2012;7:1401-1422.
8. CDC. CDC Grand Rounds: the growing threat of multidrug-resistant gonorrhea. MMWR Morb Mortal Wkly Rep. 2013;62:103-106.
9. Kidd S, Workowski KA. Management of gonorrhea in adolescents and adults in the United States. Clin Infect Dis. 2015;61(suppl 8):S785-S801.
10. World Health Organization. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. Geneva, Switzerland: World Health Organization; 2012.
11. McKie RA. Sexually transmitted diseases. www.ahcmedia.com/articles/78496-sexually-transmitted-diseases. Accessed May 1, 2016.
To comment on this article, contact rdavidson@uspharmacist.com.





