US Pharm. 2021;46(8):17-27.
Type 2 diabetes mellitus (T2DM) is a complex metabolic disorder characterized by insulin resistance and pancreatic beta-cell dysfunction, resulting in hyperinsulinemia and hyperglycemia.1 Once considered a disease primarily limited to the adult population, the incidence in children and adolescents is rising at an alarming rate, paralleling the growing childhood obesity epidemic.2-5 While its overall incidence remains lower compared with type 1 diabetes mellitus (T1DM), the number of new T2DM diagnoses in individuals younger than age 20 years has increased in all age, sex, and race/ethnicity groups (except non-Hispanic whites).5 Between 2002 and 2015, the SEARCH for Diabetes in Youth study identified 3,916 youths aged 10 to 19 years with newly diagnosed T2DM, which reflected a 4.8% increase per year.5 The incidence of childhood T2DM among racial/ethnic minorities has increased at a constant rate, with the steepest increase (per year) observed among Asians/Pacific Islanders (7.7%), followed by Hispanics (6.5%), non-Hispanic Blacks (6.0%), and Pima American Indians (3.7%).5
Pediatric T2DM is more progressive than its adult-onset counterpart. A decline in beta-cell function and diabetes-related complications develop more rapidly, and the response to pharmacotherapy may occur more slowly.6-9 Early, aggressive, and regimented interventions that optimize glycemic control are critical to delay, minimize, and/or prevent the development of acute and chronic complications that begin during childhood and persist into adulthood.10-13 The serious health complications associated with uncontrolled T2DM may lead to significant morbidity and mortality.14
The steady rise in youth-onset T2DM diagnoses will likely increase the healthcare burden and strain conventional care models.14 Projections from the SEARCH study estimate 84,000 youths aged 10 to 19 years will have T2DM by the year 2050, with a prevalence of 0.8 cases/1,000 youth.5 Pharmacists are aptly positioned to relieve stress on the healthcare system and contribute their expertise on T2DM management, as they serve as highly accessible and trusted front-line providers of patient care in a multitude of settings.15,16 Pharmacist involvement in diabetes care through educating, screening, ensuring medication adherence, and counseling on lifestyle factors has significantly improved clinical outcomes and quality of life.15-17
Etiology and Risk Factors
Abnormalities in normal glucose homeostasis (i.e., balance between insulin secretion by pancreatic beta-cells and insulin action) accompany T2DM.1,18 Impaired insulin-stimulated glucose uptake due to insulin resistance initially manifests as impaired glucose metabolism.19 As insulin resistance worsens, glucose tolerance becomes increasingly reduced, resulting in T2DM.19 Pubescent children (particularly females) normally experience increased insulin resistance and resultant hyperinsulinemia, which are induced by increased growth hormone secretion.20,21 Insulin deficiency, impaired glucose tolerance, and T2DM can develop in individuals unable to compensate by increasing pancreatic insulin secretion.21 Inadequate beta-cell insulin secretion is another hallmark feature of T2DM.1 In a vicious cycle, hyperglycemia worsens both insulin resistance and insulin secretory dysfunction, thereby augmenting the progression from impaired glucose tolerance to DM.22,23
Multiple environmental, social, and behavioral risk factors converge with genetic predisposition to endow T2DM with a heterogenous etiology.2 The difference in prevalence among specific ethnic groups, gender, and family history suggests a strong genetic component; however, the steady increase globally indicates that environmental culprits are equally important. Fueled by consumption of an obesogenic diet and physical inactivity, a disproportionate number of patients are obese.24,25 Obese children display hyperinsulinemia and substantially impaired insulin-stimulated glucose metabolism, whereby the elevated adiposity leads to increased action of proinflammatory metabolites and signaling proteins that dysregulate insulin secretion and sensitivity.26-30
Approximately half of adolescent girls with polycystic ovary syndrome (PCOS) display metabolic derangements similar to T2DM, which are exacerbated by obesity, rendering PCOS another significant risk factor and common comorbidity.31-33 Poor mental health and psychosocial factors, including depression and low socioeconomic status, contribute to disease risk and poor outcomes.34-36 Abnormal functioning of the hypothalamic-pituitary axis occurs in both T2DM and depression, whereby obesity may contribute to the development of depression or vice versa.35 Youth are also at risk for drug-induced T2DM due to the increased prescribing of antipsychotic medications.37
Exposure to maternal diabetes in utero and birth weight are additional risk factors. Intrauterine malnutrition and resultant low birth weight lead to insulin resistance, impaired pancreatic beta-cell development, and “catch-up” growth during childhood characterized by a disproportionately high amount of adipose tissue.38 Genes responsible for low birth weight also impair insulin sensitivity or secretion later in life.39,40 Elevated birth weight is also linked to T2DM, whereby fetal T2DM susceptibility alleles correlate with maternal risk alleles that promote maternal hyperglycemia, fetal hyperinsulinemia, and fetal insulin–mediated growth (i.e., macrosomia) with neonatal adiposity.39,41,42 Children born to women with gestational DM are predisposed to developing obesity, diabetes, hypertension, and dyslipidemia.41-43 Markers of insulin resistance, including HOMA-IR, BMI, waist circumference, and triglyceride levels, are elevated in children born to hyperglycemic mothers, with about 20% of affected children diagnosed by young adulthood.41-43 Breastfeeding is associated with reduced risk of T2DM in both mothers and infants and obesity in infants.44
Screening and Diagnosis
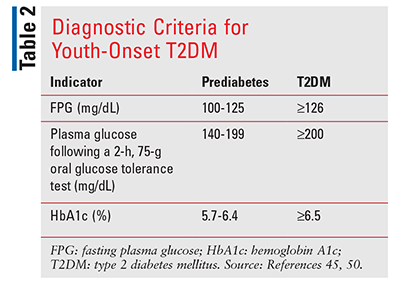
Obesity is the most important risk factor for T2DM, as many children are otherwise asymptomatic initially.6 Oftentimes, diagnosis is not made until symptoms such as polyuria, polydipsia, blurred vision, weight loss without glucosuria, and ketonuria manifest.45 Early screening is critical to identify affected individuals. Screening is based on age and BMI, with the presence of at least one additional risk factor (TABLE 1). Acanthosis nigricans, a condition that causes skin discoloration in body folds and creases, has become more common in overweight youth and is used as a cutaneous marker of insulin resistance.46 Diagnostic criteria are identical to those in adults (TABLE 2).47,48 Disagreement regarding the reliability of various testing methods exist, as they are designed for long-term outcomes and not validated in the pediatric population.49,50 Although not recommended for some patients, A1c is still generally used for diagnosis.47 Another diagnostic challenge is differentiating between T1DM and T2DM.[1] Approximately 25% of children with T1DM are obese.45 Furthermore, clinical manifestations of T1DM occur in obese T2DM patients.50 Pancreatic antibody testing differentiates autoimmune T1DM from T2DM. Accurate assessment is essential since treatment approaches, including drug regimens, nutritional recommendations, and differences in outcomes, vary depending on classification.47

Comorbidities and Complications
Insulin resistance is typically not the only metabolic derangement present at diagnosis, predisposing an individual to comorbidities such as obesity, hypertension, dyslipidemia, and nonalcoholic fatty liver disease. Baseline screenings should include blood pressure (BP), lipid panel, and liver function tests, with intermittent screening recommended thereafter.47,51 If BP is ³90th percentile for age, sex, and height or ³120/80 mm Hg for those aged 13 years or older, lifestyle modifications are first-line therapy. If BP remains elevated after 6 months or the patient initially presents with a BP ³95th percentile for age, sex, and height or ³140/90 mm Hg for those aged 13 years or older, then pharmacologic therapy with an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) is recommended. For patients with an initial low-density lipoprotein cholesterol (LDL) ³130 mg/dL or fasting triglycerides (TG) ³400 mg/dL, 6 months of lifestyle modification should be trialed prior to initiating a statin (LDL) or fibrate (TG). Specialist referral is recommended for managing additional comorbidities. T2DM is a risk factor for developing microvascular (e.g., nephropathy, neuropathy, retinopathy) and macrovascular (e.g., ischemic heart disease, peripheral vascular disease, cerebrovascular disease) complications.52 Given that the development and progression of these complications are correlated to disease duration and success of glycemic control, youth-onset T2DM confers affected patients with greater risk over their lifetime. Nephropathy, neuropathy, and retinopathy screening should be performed at the time of diagnosis and then annually.
Management
Glycemic Targets
Social determinants of health, history, and progression of T2DM, plus current therapeutic regimen should guide goals. The 2021 American Diabetes Association and 2018 International Society for Pediatric and Adolescent Diabetes guidelines provide A1c targets as surrogate markers of glucose control.47,53,54 While an A1c of <7% is currently the general target, variability is encouraged based on patient-specific factors and known morbidity and mortality outcomes related to glycemic control. A target A1c of <6.5% should be sought if achievable without increased hypoglycemic risk and/or rate of adverse events. Given that prolonged disease burden is associated with an increased risk of complications and mortality in individuals diagnosed prior to middle age, a near-normal glycemic level is ideal.21,55,56
The TODAY study determined that youth with T2DM have lower hypoglycemic rates even when taking insulin, suggesting that lower A1c goals are likely attainable.57 A less stringent target (7% or higher) may be appropriate for patients with a history of hypoglycemia, hypoglycemic unawareness, or undue burden from treatment. Self-monitoring of blood glucose (SMBG) is not standard of care for this population and should be implemented based on therapeutic regimen, feasibility, and clinical insight obtained.
Nonpharmacologic Strategies
Youth-onset T2DM is correlated to excess body weight and disproportionately affects racial minorities with disadvantaged backgrounds.58 Strategies should include diabetes education, connection to social services, and lifestyle modifications that are culturally competent. Ideally, a multidisciplinary team comprised of a physician, pharmacist, registered dietitian, psychologist, and social worker would ensure all needs are met. Diabetes education should follow a family-centered approach, as individual-level lifestyle modifications may not be sufficient or appropriate.59 Not only should education provide information on general disease state but it should also entail counseling on lifestyle modification, medical management, and mental health.
Lifestyle modifications that invoke proper nutrition and regular physical activity are foundational therapeutic approaches (TABLE 3).60 While no one diet is recommended, overarching principles on healthy eating patterns are echoed across various guidelines. Consumption of meals at regular intervals with planned snacks, decreased frequency of eating out, and portion control are also advised.58 The combination of diet and exercise improves metabolic control and favorably changes body composition.61 However, sustained lifestyle modifications are often difficult to achieve in youth, particularly older adolescents.
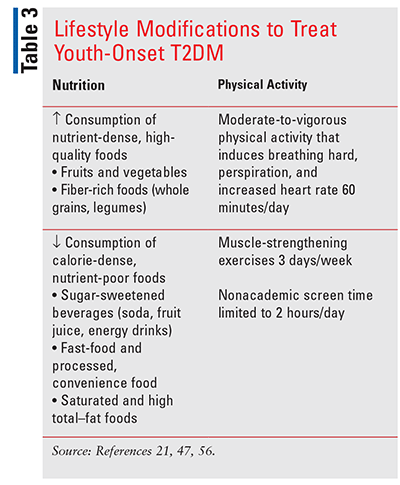
Since long-term behavioral change is the goal, it is imperative that pharmacists understand the health beliefs and behaviors of the patient/family and devise individualized, attainable goals.53 The goal for youths should be a 7% to 10% reduction in BMI for those who have completed linear growth or a BMI <85th percentile for age and sex for those who are still growing.21 Less common lifestyle modifications include smoking cessation, discontinuation of alcohol and illicit drug use, and modification of sleep behaviors to achieve a minimum of 8 hours of sleep per day. For adolescents with morbid obesity (BMI >35 kg/m2) and uncontrolled glycemia and/or serious comorbidities in whom lifestyle modifications and pharmacologic management are insufficient, metabolic surgery may be considered.
Pharmacologic Options
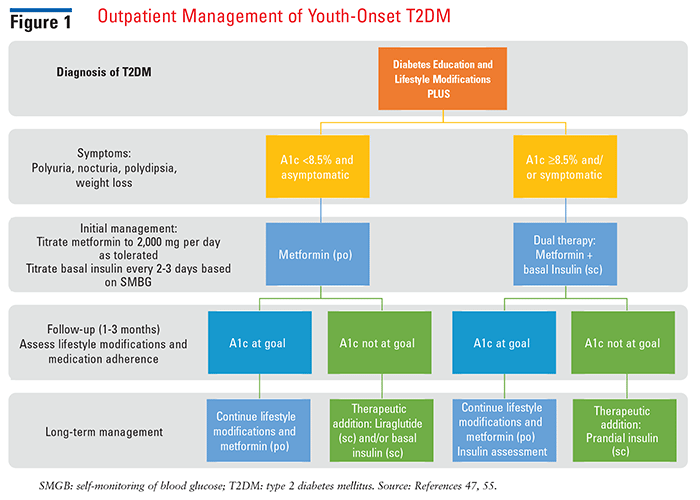
FDA-approved treatment options for youth-onset (aged 10-18 years) T2DM are currently limited to metformin (po), insulin (sc), and liraglutide (sc), which starkly contrasts the expanding list of pharmacologic agents approved for adults. With therapeutic options being sparse, agent selection and point of initiation are based on diabetes type and initial presentation. Presence of metabolic decompensation (i.e., acidosis, diabetic ketoacidosis, or hyperosmolar hyperglycemic state) is considered a medical emergency and requires insulin therapy. For confirmed or suspected T2DM, medication selection is based on initial A1c level and symptomatology (FIGURE 1).
Metformin
A naturally occurring agent known for its glucose-lowering properties since the Middle Ages, this biguanide derivative of galegine resolves hyperglycemia by reducing hepatic glucose production.62,63 Metformin, which is fairly basic and almost exclusively ionized at physiological pH, accumulates within the mitochondria of hepatocytes and inhibits the mitochondrial respiratory chain and glycerophosphate dehydrogenase.62,63 Metformin promotes intestinal glucose utilization by increasing anaerobic glucose metabolism in gut enterocytes and increases intestinal secretion of glucagon-like peptide 1 (GLP-1), which promotes insulin secretion from the pancreatic beta-cell.64 By increasing the activity of AMP-activated protein kinase (AMPK) in skeletal muscle and promoting deployment of the glucose transporter type 4 (GLUT4) and muscle glucose uptake, peripheral insulin sensitivity is also increased.65
Metformin should be initiated in conjunction with lifestyle modifications as initial therapy for all T2DM patients. Contraindications include severe biguanide allergy, renal insufficiency, or concomitant ketosis/acidosis. Safety and efficacy in pediatric patients are similar to adults, with an average fasting blood glucose decrease of 42.9 mg/dL and A1c reduction of 1% over 16 weeks.66
During the metformin monotherapy run-in phase of the TODAY trial, about 9% of participants could not tolerate metformin. Common complaints were abdominal pain, nausea, vomiting, and diarrhea. These adverse effects can be mitigated by use of extended-release formulations, administration with food, and gradual dose titration (500-mg increase/week to a max dose of 2 g/day). Due to corresponding vitamin B12 deficiency, concomitant multivitamin use is advised. While metformin poses no hypoglycemic risk and may aid in weight loss, half of youths do not experience sustained glycemic control, and beta-cell function is not improved.58
Insulin
Human insulin was the first approved product derived through recombinant technology. Numerous insulins have been formulated, yielding agents specifically geared towards optimal blood glucose control (TABLE 4).67 Similar to endogenous insulin, exogenous insulin regulates serum glucose by decreasing hepatic gluconeogenesis and increasing glucose uptake by muscle and adipose tissue.45 Most insulin formulations are approved in pediatric patients.
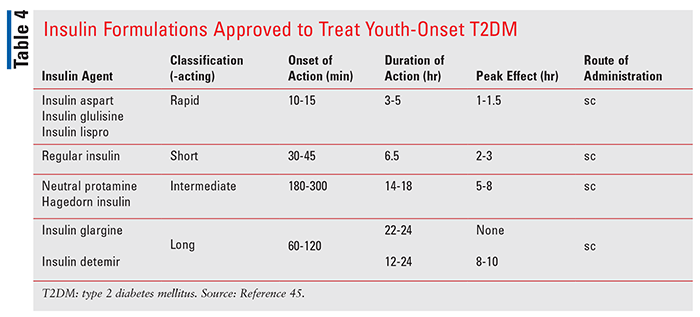
Rapid-acting: Insulin aspart, insulin glulisine, and insulin lispro each differ from regular insulin by single or double point mutations to the amino acid sequence of the B-chain (aspart: ProB28Asp; glulisine: ValB3Lys; lispro: ProB28Lys and LysB29Pro).
Short-acting: Regular insulin is “normal” recombinant human insulin with no amino acid substitutions.
Intermediate-acting: Neutral protamine Hagedorn (NPH) insulin is mixed with protamine and combined proportionally with zinc and phenol to maintain a neutral pH. This mixture forms stable crystals that extend duration of action.
Long-acting: Insulin glargine results from modifications to the A-chain (AspA21Gly) and B-chain (two arginine residues at the C-terminus) that alter the protein’s isoelectric point, causing precipitation following subcutaneous injection and forming a slow-release depot at the injection site. Insulin detemir is modified at the B-chain (removal of the B30Thr; N-acylation of the B29Lys with myristic acid), yielding a 14-carbon fatty acid side chain that affords high plasma protein binding and a circulating insulin depot.
Acute insulin therapy establishes glycemic stability and corrects metabolic derangements.21,47 In addition to patients with metabolic decompensation, it should be a part of first-line therapy for those with severe hyperglycemia (A1c ³8.5% or resting plasma blood glucose of 250 mg/dL), symptoms of hyperglycemia (i.e., polyuria, polydipsia, nocturia) and/or catabolism (i.e., weight loss), or when etiology is uncertain. Short-acting insulin is largely used in the hospital setting to rapidly correct severe hyperglycemia and metabolic decompensation. Upon stabilization, transition to a once-daily basal insulin therapy with an intermediate- or long-acting insulin is preferred.
For patients initiating insulin therapy outpatient, basal insulin sufficiently aids in achieving glycemic control during metformin titration. Standard initial dose of basal insulin is 0.5 units/kg/day, with gradual titration (10%-20%) every 2 to 3 days based on SMBG. Upon adherence to lifestyle modifications and metformin, insulin therapy can be tapered off using a reduction of 10% to 30% every few days until cessation. If glucose remains uncontrolled despite reaching maximum basal insulin levels recommended (1.5 units/kg/day), options include increasing basal insulin (with a transition to more concentrated products or division of doses) or initiation of prandial insulin. While efficacious, insulin therapy increases risk for hypoglycemia and weight gain. Diabetes education should stress compliance with alternate therapies to reduce insulin dependence.
Liraglutide: An acylated GLP-1 analogue that signals as a GLP-1 receptor (GLP1R) agonist, this incretin mimetic activates GLP1Rs in pancreatic beta-cells to evoke insulin secretion.68,69 In addition to short-term insulin release, continuous GLP1R activation promotes increased insulin synthesis and beta-cell proliferation and neogenesis.69 Liraglutide also exerts extrapancreatic effects, including antiobesity actions in the central nervous system and gastrointestinal tract.70 GLP1R distribution throughout the brain, including appetite-regulatory nuclei, mediates suppression of food intake. GLP-1 also induces satiety by delaying gastric emptying.70 These actions may be blunted in obese patients.70
Until liraglutide’s approval in 2019, insulin was the only therapeutic addition or alternative for patients unable to tolerate metformin or who required additional A1c reduction. Liraglutide was effective and safe when used in combination with metformin in the ELLIPSE trial.71 Over 13 weeks, the once-daily injection correlated to a mean A1c reduction of about 0.5%, a sustained weight reduction of about 2 kg, and a transient increase in nausea/vomiting versus placebo. No dose adjustment is required for pediatric patients: 0.6 mg once daily for 1 week followed by 1.2 mg once daily on Week 2, with a max dose of 1.8 mg achieved on Week 3. When initiating liraglutide in patients on concomitant insulin therapy, a 20% reduction in daily insulin is recommended to reduce the risk of hypoglycemia.
Therapies Under Investigation
Traditional diabetes medications have been studied in the pediatric population.21 Although efficacious, their use is not recommended due to adverse effects, particularly cardiovascular concerns with rosiglitazone, bladder cancer with pioglitazone, and weight gain with glimepiride. Therapeutic options may expand, as phase III trials for dipeptidyl peptidase-4 inhibitors (linagliptin, saxagliptin), GLP1R agonists (oral semaglutide, exenatide, dilaglutide), and sodium-glucose cotransporter-2 inhibitors (canagliflozin, empagliflozin, dapagliflozin) are ongoing.72
Conclusion
The incidence of youth-onset T2DM continues to steadily rise. Strategic interventions that control glycemia are essential to prevent and treat severe and potentially life-threatening complications in this population. Pharmacists are ideally poised to identify risk factors, provide point-of-care testing, and counsel on the prevention and effective management (pharmacologic as well as nonpharmacologic) of pediatric T2DM.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Kasuga M. Insulin resistance and pancreatic beta cell failure. J Clin Invest. 2006;116:1756-1760.
2. Reinehr T. Type 2 diabetes mellitus in children and adoles¬cents. World J Diabetes. 2013;4:270-281.
3. Kansra AR, Lakkunarajah S, Jay MS. Childhood and adolescent obesity: a review. Front Pediatr. 2021;8:581461.
4. Hamman RF, Bell RA, Dabelea D, et al. The SEARCH for Diabetes in Youth Study: rationale, findings, and future directions. Diabetes Care. 2014;37:3336-3344.
5. Dabelea D, Sauder KA, Jensen ET, et al. Twenty years of pediatric diabetes surveil¬lance: what do we know and why it matters. Ann N Y Acad Sci. 2021;1495:99-120.
6. Akhlaghi F, Matson KL, Mohammadpour AH, et al. Clinical pharmacokinetics and pharmacodynamics of antihyperglycemic medications in children and adolescents with type 2 diabetes mellitus. Clin Pharmacokinet. 2017;56:561-571.
7. Temneanu OR, Trandafir LM, Purcarea MR. Type 2 diabetes mellitus in children and adolescents: a relatively new clinical problem within pediatric practice. J Med Life. 2016;9:235-239.
8. Gungor N, Bacha F, Saad R, et al. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care. 2005;28:638-644.
9. Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY Cohort at base¬line. J Clin Endocrinol Metab. 2011;96:159-167.
10. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clinical Diabetes. 2008;26:77-82.
11. Tyagi A, Pugazhenthi S. Targeting insulin resistance to treat cognitive dysfunction. Mol Neurobiol. 2021;58:2672-2691.
12. Livshits A, Seidman DS. Fertility issues in women with diabe¬tes. Womens Health (Lond). 2009;5:701-707.
13. Frisina RD, Bazard P, Bauer M, et al. Translational implications of the interac¬tions between hormones and age-related hearing loss. Hear Res. 2021;402:108093.
14. Hirsch JD, Morello CM. Economic impact of and treatment options for type 2 diabetes. Am J Manag Care. 2017;23(suppl 13):S231-S240.
15. Hughes JD, Wibowo Y, Sunderland B, Hoti K. The role of the pharmacist in the management of type 2 diabetes: current insights and future directions. Integ Pharm Res Pract. 2017;6:15-27.
16. Brewster S, Holt R, Portlock J, Price H. The role of commu¬nity pharmacists and their position in the delivery of diabetes care: an update for medical professionals. Postgrad Med J. 2020;96:473-479.
17. Khunti N, Willis A, Davies M, Khunti K. The role of pharmacists in the management of type 2 diabetes: a literature review. Diabetes Prim Care. 2013;15:131-140.
18. Batista TM, Haider N, Kahn CR. Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia. 2021;64:994-1006.
19. Andes LJ, Cheng YJ, Rolka DB, et al. Prevalence of prediabetes among adolescents and young adults in the United States, 2005-2016. JAMA Pediatrics. 2020;174:e194498.
20. Moran A, Jacobs DR Jr, Steinberger J et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039-2044.
21. Arslanian S, Bacha F, Grey M, et al. Evaluation and management of youth-onset type 2 diabetes: a position statement by the American Diabetes Association. Diabetes Care. 2018;41:2648-2668.
22. Tomás E, Lin YS, Dagher Z, et al. Hyperglycemia and insulin resistance: possible mechanisms. Ann N Y Acad Sci. 2002;967:43-51.
23. Kawahito S, Kitahata H, Oshita S. Problems associated with glucose toxicity: role of hyperglycemia-induced oxidative stress. World J Gastroenterol. 2009;15:4137-4142.
24. Nadeau K, Dabelea D. Epidemiology of type 2 diabetes in children and adolescents. Endocrine Res. 2007;33:35-58.
25. Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Curr Diab Rep. 2014;14:508.
26. Rupérez FJ, Martos-Moreno GA, Chamoso-Sánchez D, et al. Insulin resistance in obese children: what can metabolomics and adipokine modelling contribute? Nutrients. 2020;12:3310.
27. Reinehr T, Roth CL. Inflammation markers in type 2 diabetes and the metabolic syndrome in the pediatric population. Curr Diab Rep. 2018;18:131.
28. Suliga E. Visceral adipose tissue in children and adolescents: a review. Nutr Res Rev. 2009;22:137-147.
29. Maffeis C, Morandi A. Body composition and insulin resistance in children. Eur J Clin Nutr. 2018;72:1239-1245.
30. Herrada AA, Olate-Briones A, Rojas A, et al. Adipose tissue macrophages as a thera¬peutic target in obesity-associated diseases. Obes Rev. 2021;22:e13200.
31. Marshall JC, Dunaif A. Should all women with PCOS should be treated for insulin resistance? Fertil Steril. 2012;97:18-22.
32. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981-1030.
33. Anderson AD, Solorzano CMB, McCartney CR. Childhood obesity and its impact on the development of adolescent PCOS. Semin Reprod Med. 2014;32:202-213.
34. Tsenkova V, Pudrovska T, Karlamangla A. Childhood socioeconomic disadvantage and prediabetes and diabetes in later life: a study of biopsychosocial pathways. Psychosom Med. 2014;76:622-628.
35. Lyra e Silva ND, Lam MP, Soares CN, et al. Insulin resistance as a shared pathogenic mechanism between depression and type 2 diabetes. Front Psychiatry. 2019;10:57.
36. Ducat L, Philipson LH, Anderson BJ. The mental health comorbidities of diabetes. JAMA. 2014;312:691-692.
37. Bobo W, Cooper WO, Stein CM, et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry. 2013;70:1067-1075.
38. Dulloo AG, Jacquet J, Seydoux J, Montani JP. The thrifty ‘catch-up fat’ phenotype: its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int J Obes (London). 2006;30(suppl 4):S23-S35.
39. Hattersley AT, Tooke JE. The fetal insulin hypothesis: an alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet. 1999;353:1789-1792.
40. Beaumont RN, Horikoshi M, McCarthy MI, Freathy RM. How can genetic studies help us to understand links between birth weight and type 2 diabetes? Curr Diab Rep. 2017;17:22.
41. Palatianou ME, Simos YV, Andronikou SK, Kiortsis DN. Long-term metabolic effects of high birth weight: a critical review of the literature. Horm Metab Res. 2014;46:911-920.
42. Johnsson IW, Haglund B, Ahlsson F, Gustafsson J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes. 2015;10:77-83.
43. Alejandro EU, Mamerto TP, Chung G, et al. Gestational diabetes mellitus: a harbinger of the vicious cycle of diabetes. Int J Mol Sci. 2020;21:5003.
44. Gunderson EP. Breast-feeding and diabetes: long-term impact on mothers and their infants. Curr Diab Rep. 2008;8:279-286.
45. St Onge E, Miller SA, Motycka C, DeBerry A. A review of the treatment of type 2 diabetes in children. J Pediatr Pharmacol Ther. 2015;20:4-16.
46. Maguolo A, Maffeis C. Acanthosis nigricans in childhood: a cutaneous marker that should not be underestimated, especially in obese children. Acta Paediatr. 2020;109:481-487.
47. American Diabetes Association. Children and adolescents: Standards of Medical Care in Dia-betes—2021. Diabetes Care. 2021;44(suppl 1):S180-S199.
48. Xu H, Verre MC. Type 2 diabetes mellitus in children. Am Fam Physician. 2018;98:590-594.
49. Vajravelu ME, Lee JM. Identifying prediabetes and type 2 diabetes in asymptomatic youth: should HbA1c be used as a diagnostic approach? Curr Diab Rep. 2018;18:43.
50. Kapadia C, Zeitler P. Hemoglobin A1c measurement for the diagnosis of type 2 diabetes in children. Int J Pediatr Endocrinol. 2012;2012:31.
51. Springer SC, Silverstein J, Copeland K, et al. Management of type 2 diabetes mellitus in children and adolescents. Pediatrics. 2013;131:e648-e664.
52. Arenaza L, Medrano M, Amasene M, et al. Prevention of diabetes in overweight/obese children through a family based intervention program including supervised exercise (PREDIKID Project): study protocol for a randomized controlled trial. Trials. 2017;18:372.
53. Zeitler P, Arslanian S, Fu J, et al. ISPAD Clinical Practice Consensus Guidelines 2018: type 2 diabetes mellitus in youth. Pediatr Diabetes. 2018;19(suppl 27):28-46.
54. DiMeglio LA, Acerini CL, Codner E, et al. ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabe¬tes. Pediatr Diabetes. 2018;19(suppl 27):105-114.
55. Serbis A, Giapros V, Kotanidou EP, et al. Diagnosis, treatment and prevention of type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2021;12:344-365.
56. Peña AS, Curran JA, Fuery M, et al. Screening, assessment and management of type 2 diabetes mellitus in children and adolescents: Australasian Paediatric Endocrine Group Guidelines. Med J Aust. 2020;213:30-43.
57. TODAY Study Group. Safety and tolerability of the treatment of youth-onset type 2 diabetes: the TODAY experience. Diabetes Care. 2013;36:1765-1771.
58. Singhal S, Kumar S. Current perspectives on management of type 2 diabetes in youth. Children (Basel). 2021;8:37.
59. American Association of Diabetes Educators. The role of the diabetes educator in pediatric diabetes: the etiology of the diagnosis. www.diabeteseducator.org/docs/default-source/practice/educator-tools/pediatrics/the-role-of-the-diabetes-educator-in-pediatric-diabetes—final-website.pdf?sfvrsn=2. Accessed July 26, 2021.
60. McGavock J, Dart A, Wicklow B. Lifestyle therapy for the treatment of youth with type 2 diabetes. Curr Diab Rep. 2015;15:568.
61. Kaar JL, Schmiege SJ, Drews K, et al. Evaluation of the longitudinal change in health behavior profiles across treatment groups in the TODAY clinical trial. Pediatr Diabetes. 2020;21:224-232.
62. Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochemical J. 2000;348(pt 3):607-614.
63. Bridges HR, Jones AJY, Pollak MN, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J. 2014;462:475-487.
64. Preiss D, Dawed A, Welsh P, et al. Sustained influence of metformin therapy on circulating glucagon-like peptide-1 levels in individuals with and without type 2 diabetes. Diabetes Obes Metab. 2017;19:356-363.
65. Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP-activated protein-kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074-2081.
66. Jones KL, Arslanian S, Peterokova VA, et al. Effect of met¬formin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2002;25:89-94.
67. Gururaj Setty S, Crasto W, Jarvis J, et al. New insulins and newer insulin regimens: a review of their role in improving gly¬caemic control in patients with diabetes. Postgrad Med J. 2016;92:152-164.
68. Kapodistria K, Tsilibary EP, Kotsopoulou E, et al. Liraglu¬tide, a human glucagon-like peptide-1 analogue, stimulates AKT-dependent survival signalling and inhibits pancreatic beta-cell apoptosis. J Cell Mol Med. 2018;22:2970-2980.
69. Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007;113:546-593.
70. Crane J, McGowan B. The GLP-1 agonist, liraglutide, as a pharmacotherapy for obesity. Ther Adv Chronic Dis. 2016;7:92-107.
71. Tamborlane W, Barrientos-Pérez M, Fainberg U, et al. Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med. 2019;381:637-646.
72. ClinicalTrials.gov. Youth. Type 2 Diabetes. List Results. https://clinicaltrials.gov/ct2/results?cond=Type+2+Diabetes&term=youth&cntry=&state=&city=&dist=. Accessed July 26, 2021.
To comment on this article, contact rdavidson@uspharmacist.com.





