US Pharm. 2023;48(3):17-21.
ABSTRACT: Osteoarthritis (OA) is a progressive disease in which cartilage is destroyed, resulting in painful, swollen joints. It is the most common form of arthritis, affecting millions of Americans. The American College of Rheumatology (ACR), in collaboration with the Arthritis Foundation, updated the ACR 2012 management of hand, hip, and knee OA treatment guidelines in 2019. Based on available evidence, the consensus panel updated recommendations for oral nonsteroidal anti-inflammatory drugs (NSAIDs), topical NSAIDs, duloxetine, opioids, glucosamine, and chondroitin. In understanding the new treatment guidelines, pharmacists can assist patients in finding individualized, safe, and effective treatment options.
Arthritis is a leading cause of disability, affecting nearly 23.7% of adults living in the United States.¹ Osteoarthritis (OA), the most common form of arthritis, affects an estimated 32.5 million U.S. adults. Risk factors for developing OA include age (older than age 50 years), obesity, joint injury (bone, cartilage, and ligament damage), joint overuse, and gender (female).2
OA is characterized by the progressive destruction of cartilage, primarily in the hand, hip, and knee joints. Historically thought to be a noninflammatory disease, new research suggests that synovitis and joint inflammation are found in a significant proportion of OA patients.³ Patients with OA commonly present with painful, stiff, and swollen joints, accompanied by a decreased range of motion. Deep, achy pain typically presents with motion while stiffness and decreased range of motion improve with movement. In knee and hip OA, swollen joints and weak muscles around the affected joint contribute to instability, affecting overall balance and increasing fall risk.² Diagnosis is based on symptoms, in conjunction with x-rays, MRIs, and blood tests (to rule out rheumatoid arthritis).²
ACR/AF 2019 Guideline Recommendations
The American College of Rheumatology (ACR), in collaboration with the Arthritis Foundation (AF), updated the 2012 ACR guidelines for the management of hand, hip, and knee OA in 2019.4,5 This guideline is in place to assist clinicians and patients in developing a comprehensive treatment plan that is based not only on available evidence but also on patient comorbidities, preferences, and values. There are currently no medications approved for preventing or altering the progression of OA. Pharmacologic and nonpharmacologic treatments focus on pain management and maintaining or improving quality of life and joint mobility. Symptoms are often managed using OTC or prescription analgesics or anti-inflammatory medications, in addition to nonpharmacologic treatments such as exercise, physical therapy, and weight loss.
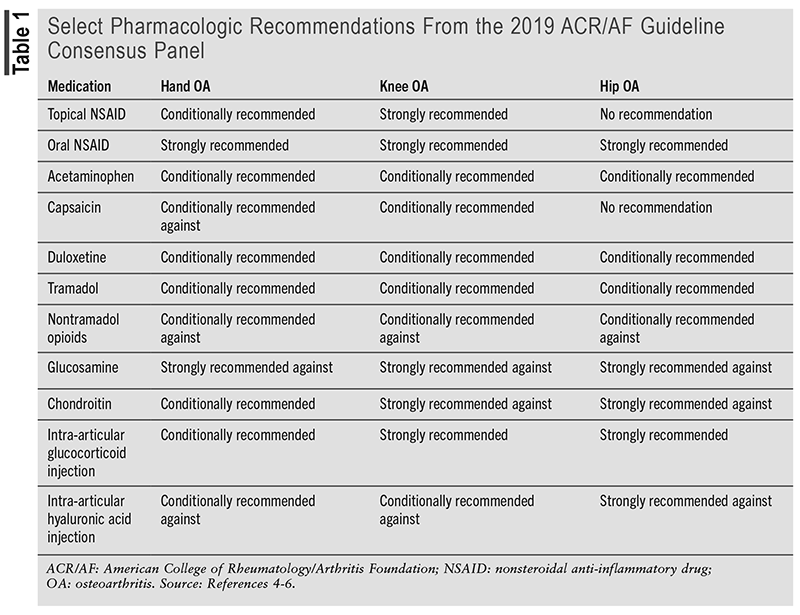
TABLE 1 provides an overview of selected pharmacologic recommendations from the 2019 ACR/AF guideline consensus panel. The panel strongly recommends topical and oral nonsteroidal anti-inflammatory drugs (NSAIDs) for knee OA and strongly recommends oral NSAIDs for hand, hip, and knee OA, which were previously conditional recommendations in the 2012 ACR guideline.4,5 Oral acetaminophen, duloxetine, tramadol, and topical capsaicin are alternative drugs conditionally recommended for patients unable to use NSAIDs. Additionally, the panel strongly recommends against the use of glucosamine and chondroitin for knee and hip OA.4 Intra-articular corticosteroid injections are strongly recommended for patients with knee or hip OA who have failed oral or topical therapy.6
Nonpharmacologic Therapies
Exercise (e.g., walking, water aerobics, strength training), weight loss, self-efficacy, and self-management programs are considered beneficial in all forms of OA. Patients are encouraged to participate in exercises they find comfortable, such as tai chi, which has been found particularly beneficial for knee and hip OA.4 Weight loss also helps reduce the stress placed on the knee and hip joints, potentially slowing the progression of the disease.4,7 Assisted devices, such as a cane, knee brace, or hand orthosis, may also be useful for some patients to reduce symptoms, improve joint function, and increase quality of life.4,8,9 Self-efficacy and self-management programs are beneficial to provide patient education about OA, medication use and side effects, and approaches and goals for exercise and weight loss.4
NSAIDs
The 2019 ACR guideline strongly recommends topical and oral NSAIDs for OA management. NSAIDs work by inhibiting cyclo-oxygenase enzymes, decreasing production of prostaglandins and prostacyclins, resulting in anti-inflammatory, antipyretic, and analgesic effects. Recent meta-analyses have shown that oral NSAIDs provide superior pain relief compared with acetaminophen, with results being most pronounced in patients with moderate-to-severe pain.10,11 Comparator trials have shown similar efficacy between oral and topical NSAIDs for knee and hand OA.12-14 Although topical NSAIDs are effective for hand OA, frequent use of hands (e.g., handwashing, typing) limits their use. Currently, there is no recommendation for the use of topical NSAIDs for hip OA due to questionable joint penetration and insufficient data.4 A notable advantage of topical NSAIDs is localized pain relief with minimal systemic absorption, which results in a reduced risk of systemic side effects.12-14 Due to comparable efficacy and improved tolerability, topical NSAIDs are recommended as first-line treatment over oral NSAIDs for knee OA.4
Oral NSAIDs are available both OTC (e.g., ibuprofen, naproxen) and by prescription (e.g., ibuprofen, naproxen, diclofenac, meloxicam, celecoxib). Currently, diclofenac is the only commercially available topical NSAID product. Diclofenac is applied topically two to four times per day and is available OTC as a 1% gel formulation and by prescription as a cream (2.5%) and solution (1.5%, 2%). When using the gel formulation, patients should be counseled to use the dosing card. It is important to note that topical NSAIDs will not provide immediate pain relief; these products take a minimum of 1 to 2 weeks to begin working.12,14
While oral NSAIDs have been proven effective for the management of OA, they are accompanied by significant side effects and contraindications, limiting their use in certain patient populations. Serious side effects include increased risk of major cardiovascular (CV) events (e.g., myocardial infarction, stroke), reduced kidney function, gastrointestinal (GI) bleeding, and fluid retention. Strong caution should be used when recommending oral NSAIDs for elderly patients and those with a history significant for reduced kidney function, impaired liver function, CV conditions (e.g., hypertension, stroke, heart failure, etc.), or GI ulcers, or those taking anticoagulant or antiplatelet medications.15-17 OA patients at high CV or GI risk may be managed using an oral NSAID after careful selection of the specific agent and close monitoring.15 For patients at high CV and GI risk, it may be best to avoid oral NSAIDs.15 It is important to note that oral NSAIDs should be used at the lowest effective dose for the shortest duration possible to limit side effects. In contrast, topical NSAIDs, when used at recommended doses, have minimal systemic side effects and are considered safe for most patients. Providers should take patients’ past medical history, current medications, and comorbidities into consideration when determining the appropriateness of oral NSAIDs.
Acetaminophen
Acetaminophen has long been considered the drug of choice for noninflammatory hip and knee OA and continues to be conditionally recommended in the 2019 ACR/AF for patients unable to take NSAIDs.4,5 Although acetaminophen is thought to benefit OA symptoms through its analgesic and weak anti-inflammatory effects, meta-analyses have determined a low to minimal level of effectiveness on pain and functional status.11,18 A systematic review by Leopoldino et al comparing the effects of paracetamol versus placebo for knee and hip OA concluded that there is a high level of evidence that paracetamol results in minimal pain relief.18 Acetaminophen is available OTC in 325-mg, 500-mg, and 650-mg strengths, with a maximum daily dose of 3 to 4 g.4,17 Although it is generally well tolerated and safe when used at recommended doses, patients with acute or chronic liver disease should avoid acetaminophen.
Duloxetine
Duloxetine is conditionally recommended for hand, knee, and hip OA and may be considered as an alternative to NSAID therapy.4 Duloxetine primarily inhibits serotonin and norepinephrine reuptake while weakly inhibiting dopamine reuptake, lessening peripheral pain signals to provide pain relief.17,19 Duloxetine has been shown to have a statistically significant benefit on the function and pain in patients who have knee OA that is comparable to patients taking prescription NSAIDs.20 Duloxetine should be initiated at 30 mg once daily for the first week, then increased to the maximum dosage of 60 mg once daily.17 Common side effects include headache, constipation, and dry mouth.17
Tramadol and Other Opioids
Tramadol is conditionally recommended in OA patients who are unable to take oral NSAIDs or continue experiencing symptoms while using NSAIDs. While the analgesic effects of tramadol have shown a mild reduction in arthritis symptoms when compared with a placebo, they are often accompanied by a number of intolerable side effects, such as constipation, dizziness, and headaches.17,21 Nontramadol opioids are not recommended due to an increased risk of side effects and abuse potential, but these may be considered if tramadol is not sufficient for pain relief.4 Chronic pain dosing for tramadol is 25 to 50 mg every 6 hours as needed, to a maximum of 400 mg daily.17 When counseling, pharmacists should educate patients on the important side effects and abuse potential associated with tramadol and nontramadol opioids.
Capsaicin
Topical capsaicin is conditionally recommended for knee OA and should be considered as an alternative to topical NSAIDs before attempting oral therapies.14 Capsaicin, a derivative of chili peppers, is thought to provide analgesic effects by stimulating the transient receptor potential vanilloid 1 receptor (TRPV1), initially causing a burning sensation followed by pain relief.17 When compared with placebo, capsaicin has been found superior in regard to pain relief for knee and hand OA.22 Similar to topical NSAIDs, capsaicin is applied topically three to four times per day and may take 2 or more weeks to reach maximal efficacy.17 The predominant side effect is burning upon application and skin irritation, which may lead to discontinuation. It is not routinely recommended for hand OA due to the potential harm the product may cause if it comes into contact with a patient’s eyes or mouth.
Glucosamine and Chondroitin
Although glucosamine and chondroitin have been used as a natural medicine to treat OA symptoms for many years, research suggests minimal to no benefit.22,23 The 2019 ACR/AF guidelines strongly recommend against the use of chondroitin and glucosamine use for hip and knee OA, while chondroitin is conditionally recommended for hand OA.4 These supplements work to promote the production and prevent the degradation of cartilage.25 A meta-analysis by Wandel et al demonstrated that neither of these products alone or in combination result in improved pain when compared with placebo.23 In regard to hand OA, a study by Gabay et al showed that chondroitin 800 mg compared with placebo significantly reduced pain using the Visual Analog Scale and improved the duration of morning stiffness and functionality; however, no additional studies have replicated these results.24 It can be noted that there is significant variation when it comes to the formulation and concentration being used, which may contribute to the conflicting efficacy found in the literature for these products. Both agents are well tolerated, but common side effects include abdominal pain and other GI-related events.25
Intra-Articular Injections
Intra-articular injections, specifically glucocorticoid and hyaluronic acid, are generally reserved as last-line therapy options due to the invasive nature and side effects associated with therapy.4 An early 2000s study indicated that intra-articular glucocorticoid injections exhibit long-term efficacy and safety when compared with placebo for the management of symptomatic knee OA.26 In contrast, a more recent study found a reduction in joint cartilage, potentially worsening pain and progression of OA when used long term.27 At this time, due to limited and conflicting evidence supporting their use for OA management, intra-articular injections with hyaluronic acid are conditionally recommended against in hand and knee OA and recommended against in hip OA.4,28
Role of the Pharmacist
When helping patients manage their OA, it is important to individualize recommendations based on disease progression, comorbidities, preferences, and values. Nonpharmacologic therapies are encouraged in conjunction with pharmacologic therapies. While capsaicin and topical NSAIDs may be useful early in disease management, the frequency of application and potential for skin irritation can be limiting. Acetaminophen is well tolerated, with few side effects; however, it is minimally effective for pain relief. Oral NSAIDs are an effective option, but due to the significant side-effect burden, long-term use may be limited. It is pertinent for pharmacists to collect a thorough and accurate medical and drug history when assisting patients with selection of OTC products to determine the most appropriate therapy.
Conclusion
OA, the most common form of arthritis, is a progressive joint disease that primarily affects the hand, hip, and knee joints. Recent research suggests that synovitis and joint inflammation accompany the destruction of joint cartilage, resulting in OA. This new information surrounding the pathophysiology of OA, in addition to greater efficacy, may further support why oral NSAIDs are recommended over acetaminophen for patients without contraindications to therapy. The 2019 updated guideline does not recommend a stepwise treatment approach. Rather, it recommends a patient-centered approach that takes patient preferences, values, and comorbidities into consideration.
REFERENCES
1. Theis K, Murphy L, Guglielmo D, et al. Prevalence of arthritis and arthritis-attributable activity limitation–United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2021;70(40):1401-1407.
2. Arthritis Foundation. Osteoarthritis. www.arthritis.org/diseases/osteoarthritis. Accessed December 10, 2022.
3. Sokolove J, Lepus CM. Role of inflammation in the pathogenenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77-94.
4. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee [published correction appears in Arthritis Rheumatol. 2021;73(5):764]. Arthritis Rheumatol. 2020;72(2):220-233.
5. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharma¬cologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
6. CDC. Osteoarthritis (OA). July 27, 2020. www.cdc.gov/arthritis/basics/osteoarthritis.htm. Accessed December 10, 2022.
7. Vincent HK, Heywood K, Connelly J, Hurley RW. Obesity and weight loss in the treatment and prevention of osteoarthritis. PM R. 2012;4(5 suppl):S59-S67.
8. Jones A, Silva PG, Silva AC, et al. Evaluation of immediate impact of cane use on energy expenditure during gait in patients with knee osteoarthritis. Gait Posture. 2012;35(3):435-439.
9. Raja K, Dewan N. Efficacy of knee braces and foot orthoses in conservative management of knee osteoarthritis. Am J Phys Med Rehabil. 2011;90(3):247-262.
10. Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;2006(1):CD004257.
11. Da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21-e33.
12. Derry S, Conaghan P, Da Silva JAP, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;4(4):CD007400.
13. Simon LS, Grierson LM, Naseer Z, et al. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis. Pain. 2009;143(3):238-245.
14. Rannou F, Pelletier JP, Martel-Pelletier J. Efficacy and safety of topical NSAIDs in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 suppl):S18-S21.
15. Bello AE, Holt RJ. Cardiovascular risk with non-steroidal anti-inflammatory drugs: clinical implications. Drug Saf. 2014;37(11): 897-902.
16. Bhala N, Emberson J, Merhi A, et al; Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomized trials. Lancet. 2013;382:769-779.
17. Lexi-Drugs. Tramadol. Updated July 27, 2015. Hudson, OH: Lexicomp, 2015. http://online.lexi.com/. Accessed December 3, 2022.
18. Leopoldino AO, Machado GC, Ferreira PH, et al. Paracetamol versus placebo for knee and hip osteoarthritis. Cochrane Database Syst Rev. 2019;2(2):CD013273.
19. Chen B, Duan J, Wen S, et al. An updated systematic review and meta-analysis of duloxetine for knee osteoarthritis. Pain. 2021;37(11):852-862.
20. Osani MC, Bannuru RR. Efficacy and safety of duloxetine in osteoarthritis: a systematic review and meta-analysis. Korean J Intern Med. 2019;34(5):966-973.
21. Toupin April K, Bisaillon J, Welch V, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;5(5):CD005522.
22. Persson MSM, Stocks J, Walsh DA, et al. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthritis Cartilage. 2018;26(12):1575-1582.
23. Wandel S, Juni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675.
24. Gabay C, Medinger-Sadowski C, Gascon D, et al. Symptomatic effects of chondroitin 4 and chondroitin 6 sulfate on hand osteoarthritis: a randomized, double-blind, placebo-controlled clinical trial at a single center. Arthritis Rheum. 2011;63(11):3383-3391.
25. Natural Medicines. Somerville, MA: Therapeutic Research Center. https://naturalmedicines.therapeuticresearch.com. Accessed December 13, 2022.
26. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee. Arthritis Rheum. 2003;48(2):370-377.
27. McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis. JAMA. 2017;317(19):1967-1975.
28. Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;5(3):351-361.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





