US Pharm. 2023;48(9):43-47.
The hepatitis B virus (HBV), an enveloped, partially double-stranded DNA virus, is a significant viral infection that causes both acute and chronic infection of the liver. HBV is primarily transmitted through blood and bodily fluids, including unprotected sexual contact, sharing of contaminated needles, and perinatal transmission from an infected mother to her child. Most immunocompetent individuals who are infected with HBV will spontaneously clear the infection within 6 months and will not need treatment. Chronic hepatitis B occurs when the HBV infection persists for more than 6 months. The likelihood of transitioning from acute to chronic infection decreases as the age at infection increases. Newborns have a roughly 90% chance of progression, while children have a 20% chance, and immunocompetent adults have a rate of less than 5%. The consequences of chronic infection can be far-reaching. Over time, it can lead to liver cirrhosis, characterized by fibrosis and scarring of the liver, impairing its function. Furthermore, chronic hepatitis B infection significantly increases the risk of developing hepatocellular carcinoma (HCC), the most common type of liver cancer.1
Hepatitis B is a vaccine-preventable disease. Recent years have seen a decline in the number of acute and chronic hepatitis B infections in all countries, especially among children and young adults, primarily due to the widespread adoption of universal infant vaccination. Despite the widespread availability of hepatitis B vaccines, outbreaks continue to happen; the combination of the opioid epidemic and low vaccination rates among adults has been linked to a rise in the incidence of acute HBV infection. Hepatitis B disease continues to be a major public health priority. This liver disease chronically affects approximately 296 million and causes 820,000 deaths worldwide. In the United States alone, approximately 862,000 people are living with this chronic liver condition, but this number may not accurately represent all cases because up to two-thirds of individuals may be unaware of their infection, putting the number of infected individuals anywhere from 850,000 to 2.2 million.1-5
Transmission and Risk Factors
HBV can be transmitted to nonimmune individuals through percutaneous or mucosal exposure to infectious biological material, such as blood, semen, and saliva. It can survive in the environment for at least 7 days and is more infectious compared with HIV. The primary modes of transmission include sexual contact, transmission from hepatitis B surface antigen (HBsAg)–positive mothers to newborns during perinatal or vertical transmission, household transmission (particularly among children) through unnoticed parenteral exposure (likely through open cuts and sores) or through the sharing of toothbrushes, razors, or other medical equipment, and transmission through contaminated syringes or needles among individuals who inject drugs. High-risk groups susceptible to HBV infection include individuals born in regions with intermediate or high prevalence, people who engage in drug injection, men who have sex with men, and individuals with HIV infection, as well as sexual partners, needle-sharing contacts, and household members of HBsAg-positive individuals, with the highest risk among unvaccinated children and sex partners of chronically infected HBV individuals.1
Screening
In March 2023, the CDC released revised guidelines concerning hepatitis B screening and testing. In these guidelines, the term “screening” pertains to the testing of individuals who are not known to have an elevated risk of being exposed to HBV and “testing” refers to the performance of serologic tests on individuals displaying symptoms or who have been identified as having an increased risk of HBV exposure. Screening is recommended for all adults aged 18 years and older at least once during their lifetime and for all pregnant women during each pregnancy, regardless of vaccination status and history of testing. Susceptible individuals with a history of risk for HBV infection, regardless of age (see TABLE 1), should be tested periodically while the risk persists. Also, anyone who is requesting HBV testing should receive it regardless of disclosure of risk.6
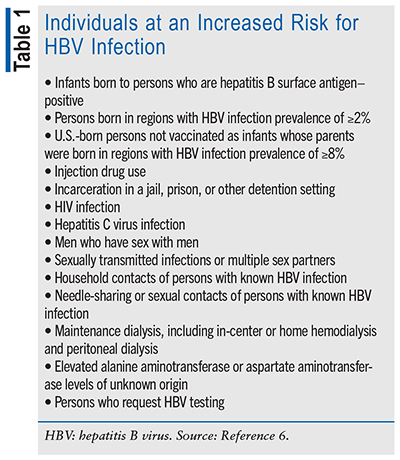
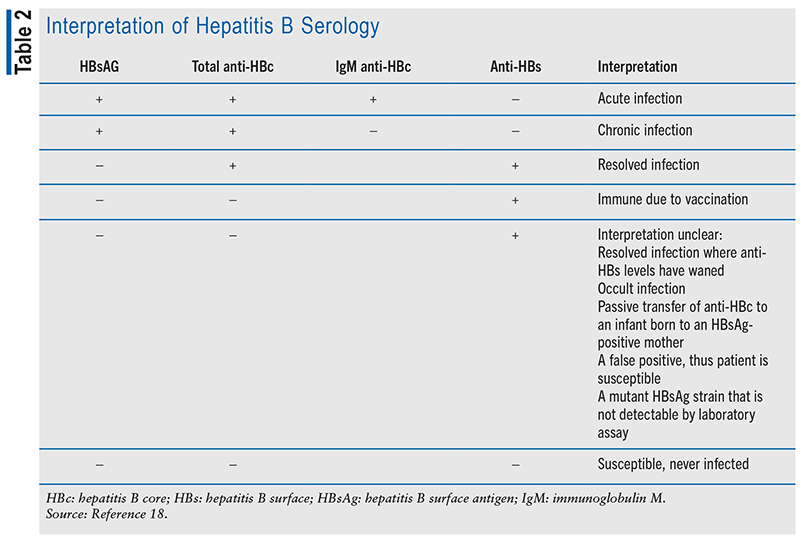
The CDC now recommends using a triple panel test to screen for hepatitis B. The triple panel test measures three different markers of hepatitis B infection: HBsAg, antibody to hepatitis B surface antigen (anti-HBs), and total antibody to hepatitis B core antigen (total anti-HBc). Different combinations of these serologic markers can indicate whether a patient has acute or chronic infection, has immunity from a previous infection or vaccination, or is susceptible to infection (see TABLE 2).
Treatment
The treatment of hepatitis B aims to achieve long-term suppression of viral replication, reduce liver inflammation, prevent disease progression, and decrease the risk of developing complications such as cirrhosis and HCC. However, it is important to note that hepatitis B treatment is not curative but only lowers the viral load in the body. The American Association for the Study of Liver Disease and the Hepatitis B Primary Care Workgroup have issued hepatitis B treatment guidance. Treatment is not warranted in all patients with chronic hepatitis B. The treatment approach for hepatitis B depends on various factors including the patient’s liver function, hepatitis B e antigen (HBeAg) status, HBsAg levels, HBV DNA levels, and the presence of liver fibrosis or cirrhosis.7,8
Treatment options for the management of chronic HBV include the use of oral antivirals (nucleoside and nucleotide analogues) or interferon-based regimens. Oral antivirals offer advantages over interferon-based therapy, such as easy administration, reliable antiviral responses, fewer side effects, and suitability for a wide range of patients, including those with decompensated liver disease. On the other hand, peginterferon alfa-2a has distinct benefits for hepatitis B treatment compared to nucleoside and nucleotide analogues: It has a fixed treatment duration, potential for long-lasting serologic and virologic responses even after therapy, and no risk of drug resistance. Factors that increase the likelihood of response to interferon-based therapy include elevated serum alanine transaminase, lower HBV DNA level, and favorable HBV genotype. However, peginterferon alfa-2a is less favored due to reasons such as subcutaneous injection, variable treatment response, and the potential for serious adverse effects, making oral antiviral therapy preferable. The preferred oral antiviral medications for initial therapy are tenofovir disoproxil, tenofovir alafenamide, and entecavir. They possess greater potency and a higher genetic barrier for resistance compared to other oral antiviral agents. The choice of specific oral antiviral medication may depend on individual patient characteristics and circumstances, including bone disease, decompensated liver disease, extrahepatic manifestations, hepatitis D virus or HIV coinfection, receipt of HIV preexposure prophylaxis, pregnancy, or renal disease. It is crucial to avoid using interferon-based treatment in individuals with decompensated liver disease, pregnant women, and those with HBV-related extrahepatic manifestations.1,7,8
Prevention
Hepatitis B vaccination is of utmost importance in preventing transmission and reducing the burden of HBV infection. Vaccination against hepatitis B has proven to be highly effective in preventing new infections, decreasing the incidence of acute and chronic hepatitis B, and reducing associated complications. It is 80% to 100% effective in preventing infection or serious illness from HBV in people who receive the complete vaccine series; however, factors such as age, sex, obesity, diabetes, smoking, chronic hepatitis C infection, alcohol use disorder, renal disease, HIV infection, celiac disease, immune compromising conditions, and certain genetic determinants can result in a suboptimal response to the vaccine. Despite high rates of vaccine acceptance among adults who are offered the hepatitis B vaccine in the U.S., overall delivery and coverage rates remain low. Approximately 30% of adults in the U.S. have been vaccinated against hepatitis B. This number has been increasing in recent years, but it is still below the CDC’s goal of 80% vaccination coverage.1,9-12
Vaccination guidelines recommend that all infants, children, adolescents aged younger than 19 years, adults aged 19 to 59 years, and adults aged 60 years and older with risk factors for HBV should receive the hepatitis B vaccine. However, adults aged 60 years and older without risk factors may also choose to receive the vaccine. Prevaccination serologic testing is generally not necessary unless the individual is at high risk for HBV infection.12,13
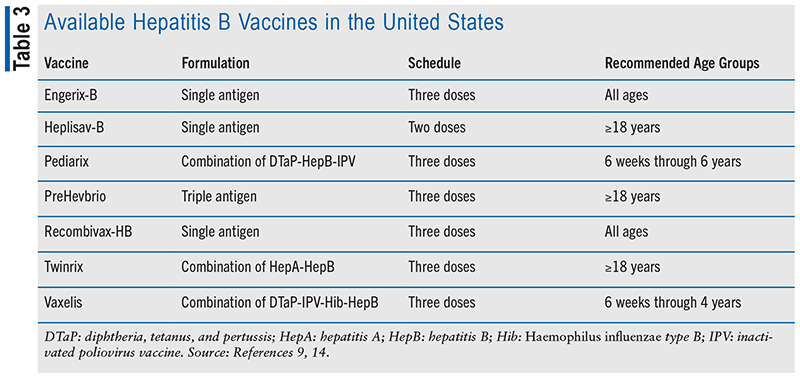
Several approved hepatitis B vaccines are available (see TABLE 3). The Advisory Committee on Immunization Practices does not provide specific guidance on vaccine preference, except that Heplisav-B and PreHevbrio should not be administered to pregnant women. The single-antigen hepatitis B vaccines, Engerix-B and Recombivax-HB, can be used interchangeably in adults. For infants, single-antigen HBV vaccines are recommended for the birth dose, while combination vaccines can be used to complete the series. Postvaccination serologic testing is recommended for specific groups, including infants born to HBsAg-positive or unknown-status mothers, healthcare workers, public safety workers at risk of exposure to blood or body fluids, individuals on hemodialysis, persons with HIV, immunocompromised individuals, and sex partners of HBsAg-positive persons. For individuals who do not develop a protective antibody response after the initial three-dose series, a fourth dose or a second full three-dose revaccination series can be considered, achieving a protective response in at least 50% of cases.9,12,14
The Pharmacist’s Role
As healthcare professionals at the forefront of patient care, pharmacists play a crucial role in the management of hepatitis B. Their involvement includes various aspects, such as medication management, patient education, adherence support, monitoring, and collaboration with other healthcare professionals. Through the provision of medication therapy management, pharmacists are responsible for ensuring appropriate medication selection, dosing, and monitoring for patients with hepatitis B in collaboration with other healthcare team members. They review medication profiles to identify potential drug-drug interactions, assess liver function, and adjust medication regimens as needed. Pharmacists also provide counseling on the proper use of antiviral medications and potential side effects. Pharmacists play a vital role in educating patients about hepatitis B, its transmission, prevention strategies, and the importance of adherence to antiviral therapy. Achieving and maintaining medication adherence are crucial for the successful management of hepatitis B.
Pharmacists work with patients to address barriers to adherence, such as medication cost, complex dosing schedules, and side effects. They provide counseling on the importance of consistent medication use and offer strategies to improve adherence, such as pill organizers, reminder systems, and medication synchronization programs. Additionally, pharmacists can provide information on lifestyle modifications, such as avoiding alcohol and certain medications that can further damage the liver. Pharmacists can address patient concerns, provide support, and empower patients to take an active role in their treatment. Most importantly, however, pharmacists play a crucial role in hepatitis B prevention through vaccination efforts by providing education, administration, and advocacy for vaccination. Their involvement helps improve access to vaccination services, enhances public awareness, and contributes to achieving higher vaccination coverage rates. The CDC recognizes pharmacists as immunizers, and they have been identified as stakeholders to help mitigate the hepatitis B crisis.15-17
Conclusion
Hepatitis B remains a significant global health concern that demands continued attention and concerted efforts. This viral infection poses risks to individuals worldwide, leading to liver damage, chronic conditions, and even mortality. However, advancements in medical science and the role of pharmacists have led to significant strides in identifying, preventing, and treating hepatitis B. Pharmacists play a pivotal role in raising awareness, educating patients, and promoting preventive measures such as vaccination. They can also contribute to early detection through screening initiatives and counseling individuals at risk. Moreover, pharmacists are instrumental in managing hepatitis B through medication adherence support, simplifying regimens, and exploring patient-assistance programs for improved accessibility and affordability. Collaboration between healthcare professionals, policymakers, and the community is crucial in combating hepatitis B. Continued research, vaccination campaigns, and public health interventions are vital in reducing the burden of this disease. By working together, pharmacists can strive for a future where hepatitis B is effectively controlled and individuals can lead healthier lives, free from the devastating consequences of this viral infection.
What Is Hepatitis B?
Hepatitis B is a viral infection caused by the hepatitis B virus. It infects the liver and can lead to inflammation, liver damage, and other complications. This virus causes both acute and chronic diseases.
How Does Hepatitis B Spread?
The virus is transmitted through contact with the blood, semen, vaginal fluids, and other body fluids of an infected person. It can be transmitted through unprotected sex, sharing needles or syringes, mother-to-child transmission during childbirth, or contact with contaminated objects. Hepatitis B is not spread through kissing, holding hands, hugging, coughing, or sneezing. It is also not spread by contaminated water or food.
What Are the Symptoms of Hepatitis B?
Many people with acute hepatitis B may not experience any symptoms. However, some individuals may develop symptoms such as fatigue, loss of appetite, nausea, jaundice (yellowing of the skin and eyes), dark urine, and abdominal pain. Chronic hepatitis B infection may not cause noticeable symptoms initially but can lead to long-term liver problems if left untreated.
How Is Hepatitis B Treated?
Doctors usually advise individuals with acute hepatitis B to rest, maintain a nutritious diet, and stay hydrated. Individuals with chronic hepatitis B should undergo evaluations for liver issues and receive regular monitoring. There are treatments available that can slow or even prevent the progression of liver disease.
Can I Prevent Hepatitis B?
The hepatitis B vaccine is the most effective way to prevent hepatitis B infection. It is administered as a series of two to four shots, depending on the vaccine type. Vaccination is recommended for all infants, children, and adults who have not been previously vaccinated. You need to receive all shots in the series for long-term protection.
Hepatitis B can also be prevented by avoiding contact with contaminated blood and unprotected sexual exposure. If you use needles for any reason, ensure that they are sterile and not shared with others. This applies to medical procedures, tattoos, body piercings, or drug use. If injecting drugs, seek help from healthcare professionals for needle exchange programs and assistance with quitting drug use.
Where Can I Go for More Information?
CDC: www.cdc.gov/hepatitis/hbv/patienteduhbv.htm
Hepatitis B Foundation: www.hepb.org/research-and-programs/hepdeltaconnect/resources/
REFERENCES
1. Jeng WJ, Papatheodoridis GV, Lok ASF. Hepatitis B. Lancet. 2023;401(10381):1039-1052.2. Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380(21):2041-2050.
3. Patel EU, Thio CL, Boon D, et al. Prevalence of hepatitis B and hepatitis D virus infections in the United States, 2011-2016. Clin Infect Dis. 2019;69(4):709-712.4. Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology. 2016;63(2):388-397.
5. Kim HS, Yang JD, El-Serag HB, Kanwal F. Awareness of chronic viral hepatitis in the United States: an update from the National Health and Nutrition Examination Survey. J Viral Hepat. 2019;26(5):596-602.6. Conners EE. Screening and testing for hepatitis B virus infection: CDC recommendations—United States, 2023. MMWR Recomm Rep. 2023;72.
7. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560-1599.8. Hepatitis B Online. Hepatitis B management: guidance for the primary care provider. February 25, 2020. www.hepatitisb.uw.edu/page/primary-care-workgroup/guidance. Accessed June 18, 2023.
9. Haber P, Schillie S. Hepatitis B. CDC. September 22, 2022. www.cdc.gov/vaccines/pubs/pinkbook/hepb.html. Accessed June 18, 2023.10. Lu PJ, Hung MC, Srivastav A, et al. Surveillance of vaccination coverage among adult populations—United States, 2018. MMWR Surveill Summ. 2021;70(3):1-26.
11. Hollinger FB. Factors influencing the immune response to hepatitis B vaccine, booster dose guidelines, and vaccine protocol recommendations. Am J Med. 1989;87(3):S36-S40.12. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67(1):1-31.
13. Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 19-59 years: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(13):477-483.14. CDC. ACIP evidence to recommendations for use of PreHevbrio hepatitis B (HepB) vaccine in adults. May 11, 2023. www.cdc.gov/vaccines/acip/recs/grade/prehevbrio-hepb-etr.html. Accessed June 18, 2023.
15. Bach AT, Goad JA. The role of community pharmacy-based vaccination in the USA: current practice and future directions. Integr Pharm Res Pract. 2015;4:67-77.16. Freeland C, Ventricelli DJ. The role of the pharmacist in preventing hepatitis b in the context of the opioid crisis. Prev Chronic Dis. 2020;17:E88.
17. United States Department of Health and Human Services. Viral hepatitis national strategic plan overview. April 22, 2016. www.hhs.gov/hepatitis/viral-hepatitis-national-strategic-plan/national-viral-hepatitis-action-plan-overview/index.html. Accessed June 18, 2023.18. CDC. Interpretation of hepatitis B serologic test results. March 3, 2023. www.cdc.gov/hepatitis/hbv/interpretationOfHepBSerologicResults.htm. Accessed June 18, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






