US Pharm. 2022;11(47):HS2-HS10.
ABSTRACT: Hyperglycemia complicates the care of non–critically ill hospitalized patients and has been associated with worse outcomes compared with hospitalized patients without hyperglycemia. In 2022, both the American Diabetes Association and the Endocrine Society released guidelines on insulin and noninsulin management, glucose-monitoring techniques, and the use of home insulin pumps. Although some controversy remains, both guidelines support using scheduled basal regimens with supplemental correctional insulin in most patients with hyperglycemia, whether or not they have diabetes. The use of noninsulin products in hospitalized patients continues to evolve, with dipeptidyl peptidase-4 inhibitors providing benefit for selected patients with diabetes. Pharmacists are key participants in therapy management to improve glucose control in non–critically ill hospitalized patients.
If improperly managed, hyperglycemia in non–critically ill hospitalized patients with or without diabetes results in increased morbidity, mortality, and hospital costs.1,2 Non–critically ill patients are those admitted for elective surgery or to the medical wards rather than the ICU. Hyperglycemia can occur in patients with type 1 diabetes (T1D), type 2 diabetes (T2D), or prediabetes; additionally, patients with or without diabetes can develop stress hyperglycemia or medication-induced hyperglycemia. Although IV insulin is recommended for critically ill patients, the preferred insulin regimen for non–critically ill patients has yet to be determined. In addition, certain noninsulin regimens may be beneficial in selected patients with diabetes. The COVID-19 pandemic necessitated therapy optimization and remote monitoring of glucose.3 The American Diabetes Association (ADA) Standards of Medical Care in Diabetes—2022 and the 2022 Endocrine Society Clinical Practice Guideline provide recommendations for management of hyperglycemia in patients hospitalized for noncritical illnesses.1,2 This article will discuss hyperglycemia management in adult patients.
ETIOLOGY OF HYPERGLYCEMIA IN HOSPITALIZED PATIENTS
Many factors influence glucose control, including nutritional status, medications, control of diabetes, and—specific to surgery—the type of anesthesia administered. General anesthesia is associated with greater insulin resistance compared with local and regional anesthesia.4 Surgery provokes a stress response in the body by inducing sympathetic nervous system stimulation and subsequent increases in catecholamines, cortisol, glucagon, and growth-hormone levels, leading to exogenous glucose production and hyperglycemia.4 Patients hospitalized for noncritical illnesses may also experience stress-related hyperglycemia caused by acute metabolic stress or by certain procedures.
GLYCEMIC TARGETS
Hyperglycemia in the non–critically ill hospitalized patient is defined as a blood glucose concentration (BGC) >140 mg/dL. According to the Endocrine Society guideline, the target glucose range for most patients with or without diabetes is 100 mg/dL to 180 mg/dL.2 The ADA recommends a target glucose range of 140 mg/dL to 180 mg/dL for patients with diabetes.1 However, these targets require consideration of the patient’s clinical situation. A higher target range may be necessary in patients with terminal illness or limited life expectancy or those at risk for hypoglycemia.1
In patients with diabetes who are undergoing elective surgery, a preoperative A1C target of <8% has been shown to decrease length of hospital stay and reduce rates of postoperative infections, respiratory complications, neurologic complications, postoperative renal failure, and cardiac complications.2 If this is not possible, a BGC of 80 mg/dL to 180 mg/dL 1 to 4 hours prior to elective surgery should be targeted.1,2
PHARMACOLOGIC MANAGEMENT OF HYPERGLYCEMIA
Insulin Therapy
Scheduled subcutaneous insulin therapy is the backbone of treatment for non–critically ill hospitalized patients with hyperglycemia. Components of insulin administration include2:
• Basal insulin—Typically, once-daily injections of long-acting or intermediate-acting insulin intended to correct fasting hyperglycemia and fulfill basal needs.
• Prandial (bolus) insulin—Rapid insulin injections to prevent postprandial hyperglycemia, generally given with basal insulin.
• Scheduled insulin—Combination of intermediate-acting or long-acting insulin with prandial or correction insulin given before meals or every 4 to 6 hours.
• Correctional insulin—Extra rapid-acting or regular insulin doses to extra insulin doses used to correct hyperglycemia, typically given in addition to scheduled doses of basal and/or prandial insulin. Correctional insulin is also known as sliding-scale insulin.2
• Basal-bolus insulin (BBI)—Combination of basal insulin given once or twice daily and prandial insulin plus correctional insulin.
Patients Without Diabetes: In these patients, correctional insulin may be considered for BGC >140 mg/dL.2 Patients with two BGC >180 mg/dL (persistent hyperglycemia) should be started on basal insulin.2
Patients Diagnosed With Diabetes: Patients with diabetes previously treated with diet or oral antidiabetic agents who are hospitalized for a noncritical illness most likely will require insulin.1 The ADA recommends that the patient’s nutritional status be considered in the selection of an insulin regimen. Non–critically ill hospitalized patients with poor oral intake and those taking nothing by mouth (NPO) should begin with basal insulin or basal plus correctional insulin, whereas an insulin regimen with basal, prandial, and correctional components aligned to meals is recommended in patients with good nutritional intake.1 Although the Endocrine Society recommends scheduled or correctional insulin, the ADA states that correctional insulin given alone be avoided in most patients with diabetes.1,2 Patients with T1D require a regimen with basal and correctional components, with additional doses of prandial insulin if the patient is eating.1
Generally, hospitalized patients with diabetes should continue home insulin therapy.1,5 Patients who were receiving insulin via a pump or self-injecting insulin pen prior to admission should continue therapy.1,2 This includes carbohydrate counting with fixed prandial insulin dosing, as applicable. TABLE 1 summarizes inpatient hyperglycemia treatment.
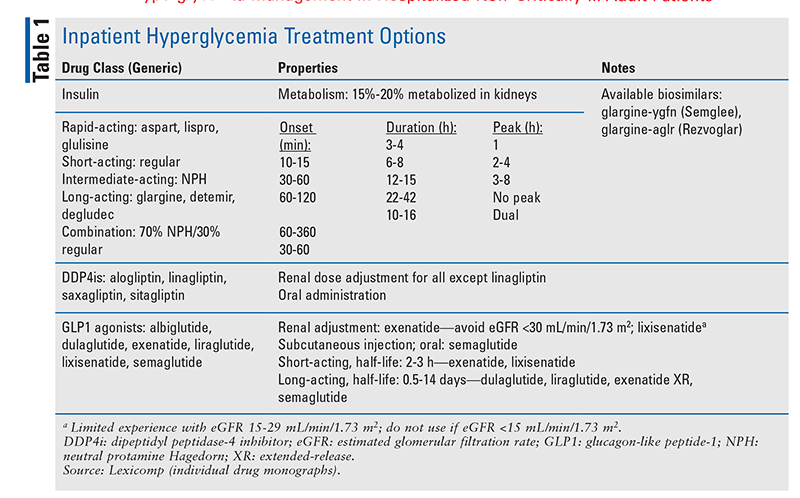
Dosing: Basal insulin 0.2 or 0.3 units/kg/day should be initiated.1 A lower starting dosage of 0.15 units/kg/day may be considered in patients with a high risk of hypoglycemia (discussed below).5 For BBI, half of the total daily dose should be basal insulin and the other half correctional (or prandial rapid-acting insulin).5 Patients on high total daily doses of insulin (>0.6 units/kg/day) likely will require a 20% reduction upon hospitalization.2 Intermediate-acting insulin and mixed insulin should be avoided in most patients.
Complications
Hypoglycemia: Hypoglycemia (BGC <70 mg/dL) may lead to neurologic or ischemic events, longer length of hospital stay, and an increased overall mortality risk.1 The risk of hypoglycemia increases during hospitalization and acute illness because of variability in insulin sensitivity, changes in hormonal responses to procedures or illness, and interruptions in usual nutritional intake.1,5 Factors associated with hypoglycemic events include older age, greater illness severity, diabetes, and the use of oral glucose-lowering medications and insulin.
Patients at high risk for developing hypoglycemia while on insulin therapy include age 65 years and older; BMI 27 kg/m2 or less; total daily dose of insulin 0.6 units/kg or higher; history of stage 3 or higher chronic kidney disease (estimated glomerular filtration rate <60 mL/min/1.73 m2); liver failure, cerebrovascular accident, active malignancy, pancreatic disorders, congestive heart failure, or infection; and history of preadmission hypoglycemia or hypoglycemia occurring during a recent or current hospitalization, or impaired awareness of hypoglycemia.2
Management addressing the increased risk of hypoglycemic events is critical. For BGC 70 mg/dL to 100 mg/dL, this might include reducing the total daily insulin dose by 20% after confirmation of the hypoglycemic event.3 It is also recommended that a nurse-initiated hypoglycemia-management protocol be in place to enable immediate treatment.1
BG Monitoring
BG monitoring aids in appropriate insulin dosing, attainment of target BGC, and prevention of hypoglycemia. BGC has traditionally been monitored through capillary point-of-care (POC) testing via BG monitors with finger sticks performed before meals and at bedtime in patients who are eating, or every 4 to 6 hours in patients taking NPO.6
Continuous glucose monitoring (CGM) technology measures glucose concentrations through a sensor that transmits results directly to a smartphone or tablet.3 During the COVID-19 pandemic, interest in CGM grew because this method eliminated the need for bedside testing and reduced the burden of care.3,7 Preliminary evidence in hospitalized patients suggests that CGM results in increased detection of hypoglycemic events and that it may be especially beneficial in patients at high risk for hypoglycemia and those with T1D.3 Although evidence supporting its use in hospitalized patients is limited, guidelines recommend CGM in patients with diabetes who are hospitalized for a noncritical illness.1,2 CGM with confirmatory bedside POC glucose monitoring is recommended in patients with diabetes at high risk for developing hypoglycemia.2 CGM may be less effective in patients with skin infections, hypoperfusion, or hypovolemia and in those receiving vasoactive medications.2
Noninsulin Therapy
Patients With Diabetes: Although insulin remains preferred therapy for inpatient hyperglycemia management, some patients with diabetes maintained on oral antidiabetic agents who are admitted to the hospital may continue therapy.1,2,8 These patients and patients admitted for elective surgery should receive a medication review, as oral antidiabetic agents are not without risk. Sulfonylureas increase the risk of hypoglycemia, especially in elderly individuals and patients with poor renal function.4 Metformin should be avoided in patients at risk for lactic acidosis, including those with renal and hepatic dysfunction and those receiving iodinated contrast. Hypoglycemia from thiazolidinediones can take several weeks to develop; furthermore, these drugs are contraindicated in patients with heart failure, which is a complication of T2D.5,6 The ADA guidelines recommend avoiding sodium-glucose cotransporter 2 inhibitors based on a lack of data and increased risk of euglycemic diabetic ketoacidosis (DKA) and genitourinary infections.1,5 Although glucagon-like peptide-1 receptor agonists reduce hypoglycemia when given in combination with insulin, they cause severe nausea and vomiting, precluding their use. TABLE 2 summarizes oral antidiabetic therapy in the surgical patient.
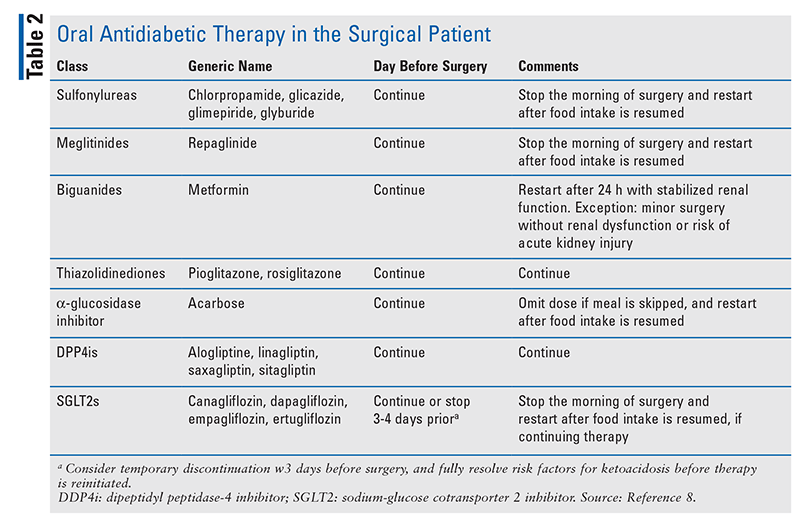
Based on emerging evidence, the Endocrine Society recommends that some patients with moderately controlled T2D (recent A1C <7.5%, BGC <180 mg/dL, total daily insulin dose <0.6 units/kg/day if on insulin prior to hospitalization) receive dipeptidyl peptidase-4 inhibitors for inpatient hyperglycemia management, in combination with correctional or scheduled insulin therapy.1,2 Continuance of any new therapies must be discussed with the patient prior to discharge.
SPECIAL SITUATIONS
Home Insulin Pumps: The insulin pump (i.e., continuous subcutaneous insulin infusion) is an increasingly popular method for diabetes management in the outpatient setting. When these patients are admitted to the hospital, confusion among healthcare providers on insulin-pump management can ensue, leading to decreased patient satisfaction. It is important to establish standardized protocols or order sets to improve insulin-pump management in the hospital. Evidence indicates similar glycemic outcomes and hypoglycemia incidences in patients continuing their insulin pump and those switched to subcutaneous insulin, suggesting that this is a safe alternative for inpatient management.9 Patients with an impaired level of consciousness, inability to appropriately adjust pump settings, critical illness, DKA, or hyperosmolar hyperglycemic state should be converted to a subcutaneous insulin regimen.2
Enteral/Parenteral Feeding: Hyperglycemia occurs in up to 30% of patients receiving enteral nutrition and more than 50% of patients receiving parenteral nutrition.10 Determining an appropriate insulin regimen for patients on nutrition support to manage hyperglycemia and prevent hypoglycemic episodes can be challenging. Guidelines recommend either a neutral protamine Hagedorn (NPH) regimen or a BBI regimen for patients on enteral nutrition.1,2 In patients receiving total parenteral nutrition (TPN), it is reasonable to add insulin to the bag or administer separate insulin injections. A recent trial found similar glycemic control, with fewer hypoglycemic episodes, when the total daily dose of insulin was added to the TPN bag.11 The ADA recommends adding 1 unit of regular insulin for every 10 g dextrose to the TPN, with supplemental correctional insulin to prevent hypoglycemia.1
Glucocorticoid-Induced Hyperglycemia (GIH): Glucocorticoids, which are used in >10% of hospitalized patients, can cause new-onset hyperglycemia or worsen glucose control in patients with preexisting diabetes.12 GIH is also associated with increased risks of mortality, cardiovascular events, and infections.2 Management of GIH should be based on the glucocorticoid being used and the dosing frequency. Guidelines recommend both NPH and BBI regimens for management, as evidence suggests similar overall outcomes.13 A reasonable approach is to use insulin that has pharmacokinetic properties similar to the prescribed glucocorticoid. In patients on shorter-acting once-daily steroids (e.g., prednisone), which peak at around 4 to 6 hours, NPH has been shown to provide similar glycemic control but lower total daily insulin requirements. NPH may be initiated at a dosage of 0.1 units/kg for every 10 mg of prednisone, up to a maximum of 0.4 units/kg.14 This regimen may be added to a BBI if a patient is already stable on the current regimen.2 For patients receiving glucocorticoids with a longer half-life, such as dexamethasone or multiple daily doses, BBI is a reasonable alternative.1,14 It is important to monitor for discontinuation or tapering of glucocorticoid therapy, as the additional insulin should be removed once the course of therapy has been completed in order to avoid hypoglycemia.
THE PHARMACIST’S ROLE
Optimal care of the hospitalized patient with hyperglycemia requires a multidisciplinary team. Through medication therapy management, pharmacists can identify whether patients are taking other medications that cause hyperglycemia or put them at risk for hypoglycemia. An advanced search in Lexicomp revealed more than 300 drugs with hyperglycemia listed under adverse events.15 Pharmacists can also play a crucial role in CGM management. In addition to assisting with implementing CGM technology, pharmacists can help monitor individual patients’ BGC. Any medications stopped before surgery should be restarted before discharge.
CONCLUSION
Strategies for glucose management in hospitalized non–critically ill patients remain controversial, with varying consensus among major guidelines. Although they can fluctuate based on patient-specific factors, glycemic targets should be used to reduce complications associated with hyperglycemia and prevent hypoglycemic episodes. Subcutaneous insulin regimens are recommended for patients with and without diabetes who experience hyperglycemia, and scheduled insulin regimens are preferred over correctional insulin. Other insulin forms, such as NPH, may be used as an alternative to long-acting insulin products in selected patients with GIH or hyperglycemia associated with enteral nutrition. More evidence is needed before noninsulin therapies can be routinely recommended. Pharmacists continue to play an integral role in selecting medication regimens to improve safety and efficacy for inpatient glucose management in the non–critically ill patient.
REFERENCES
1. American Diabetes Association Professional Practice Committee; Draznin B, Aroda VR, Bakris G, et al. 16. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S244-S253.
2. Korytkowski MT, Muniyappa R, Antinori-Lent K, et al. Management of hyperglycemia in hospitalized adult patients in non-critical care settings: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2022;107(8):2101-2128.
3. Citlalli Perez-Guzman M, Shang T, Zhang JY, et al. Continuous glucose monitoring in the hospital. Endocrinol Metab (Seoul). 2021;36(2):240-255.
4. Vogt AP, Bally L. Perioperative glucose management: current status and future directions. Best Pract Res Clin Anaesthesiol. 2020;34(2):213-224.
5. Pasquel FJ, Lansang MC, Dhatariya K, Umpierrez GE. Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol. 2021;9(3):174-188.
6. Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38.
7. Pasquel FJ, Fayfman M, Umpierrez GE. Debate on insulin vs non-insulin use in the hospital setting—is it time to revise the guidelines for the management of inpatient diabetes? Curr Diab Rep. 2019;19(9):65.
8. Preiser JC, Provenzano B, Mongkolpun W, et al. Perioperative management of oral glucose-lowering drugs in the patient with type 2 diabetes. Anesthesiology. 2020;133(2):430-438.
9. Kannan S, Satra A, Calogeras E, et al. Insulin pump patient characteristics and glucose control in the hospitalized setting. J Diabetes Sci Technol. 2014;8(3):473-478.
10. Gosmanov AR, Umpierrez GE. Management of hyperglycemia during enteral and parenteral nutrition therapy. Curr Diab Rep. 2013;13(1):155-162.
11. Olveira G, Abuin-Fernández J. Regular insulin added to total parenteral nutrition vs subcutaneous glargine in non-critically ill diabetic inpatients, a multicenter randomized clinical trial: INSUPAR trial. Clin Nutr. 2021;40(3):1440.
12. Roberts A, James J, Dhatariya K, Joint British Diabetes Societies (JBDS) for Inpatient Care. Management of hyperglycaemia and steroid (glucocorticoid) therapy: a guideline from the Joint British Diabetes Societies (JBDS) for Inpatient Care group. Diabet Med. 2018;35(8):1011-1017.
13. Ruiz de Adana MS, Colomo N, Maldonado-Araque C, et al. Randomized clinical trial of the efficacy and safety of insulin glargine vs. NPH insulin as basal insulin for the treatment of glucocorticoid induced hyperglycemia using continuous glucose monitoring in hospitalized patients with type 2 diabetes and respiratory disease. Diabetes Res Clin Pract. 2015;110(2):158-165.
14. Wallace MD, Metzger NL. Optimizing the treatment of steroid-induced hyperglycemia. Ann Pharmacother. 2018;52(1):86-90.
15. Lexicomp. Search results for “hyperglycemia” in Adverse Reactions. https://online-lexi-com.dml.regis.edu/lco/action/search?q=hyperglycemia&t=adversereactions&acs=false&db=patch_f. Accessed October 19, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





