US Pharm. 2017;42(5)HS27-HS31.
Hypophosphatasia is a rare autosomal dominant or recessive metabolic disorder caused by mutations in the alkaline phosphatase (ALPL) gene.1 It was first detected by Rathbun in 1948 and is caused by mutations in the liver/bone/kidney alkaline phosphatase gene–encoding tissue nonspecific alkaline phosphatase (TNSALP).2 To date, over 200 different mutations in ALPL have been identified.1,3 The inherited disorder results in defective mineralization of bones and/or teeth in the presence of low serum and bone alkaline phosphatase activity.2,4 The disease severity varies widely, with severe forms usually manifesting during the perinatal and/or infantile periods; mild forms are sometimes diagnosed in adulthood or remain undiagnosed.1
Epidemiology
One of the first studies dealing with the epidemiology of hypophosphatasia examined the severe form of this condition in the Hospital for Sick Children in Toronto and found the prevalence to be about 1/100,000 patients.5 The prevalence tends to be higher in the Canadian Mennonite population, perhaps as high as 1/2,500 due to the founder effect.6-8 The founder effect is defined as the loss of genetic variation that occurs when a new population is established by a very small number of individuals from a larger population, leading to a concentration of a particular type of gene. The occurrence of hyphophosphatasia due to the homozygous mutation in the Japanese population tends to be relatively high, around 1/900,000, while that in African Americans seems to be extremely rare.9,10 A survey conducted in the European population showed that the severe form of hypophosphatasia occurs in approximately 1/300,000 individuals.11Both sexes seem to be affected equally, and the burden of the illness in adulthood and childhood tends to be high.3,12
Pathophysiology
TNSALP is a phosphomonoesterase of 507 residues and is anchored at its carboxyl terminus to the plasma membrane by phosphatidylinositol-glycan moiety.3 In skeletal- muscle tissue, TNSALP works to break down mineralization inhibitors, primarily pyrophosphatase. Furthermore, it plays a role in the balance of minerals and mineralization inhibitors that are required to ensure that mineralization occurs in a temporal and spatial manner.13
Additionally, it is believed that the phosphate generated from pyrophosphate is used to ensure that hypertrophic chondrocytes undergo apoptosis as part of the coordinated changes required for endochondral ossification during growth and the repair of fractures.13 Deficient TNSALP activity, such as that seen in hypophosphatasia, leads to the extracellular accumulation of its natural substances, including inorganic pyrophosphate and pyridoxal-5-phosphate.14
Inorganic pyrophosphate is a potent inhibitor of mineralization; excesses lead to a blockage in the formation of hydroxyapatite crystal formation within the skeletal matrix, leading to rickets during growth, or osteomalacia in adults.14
The removal of pyridoxal-5-phosphate in the central nervous system allows the movement of pyridoxal across membranes.13 Impaired hydrolysis of pyridoxal-5-phosphate in severely affected infants can lead to neurotransmitter deficiency and vitamin B6 (pyridoxine)-dependent seizures.14
Clinical Forms of Hypophosphatasia
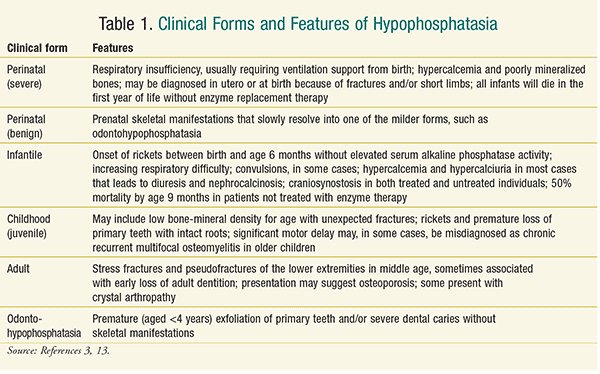
The clinical forms of hypophosphatasia are classified into six categories, based either on whether or not there are dental complications alone (odontohypophosphatasia), or on the age of the patient when skeletal and other problems first manifest.14 Table 1 describes the features of these clinical forms.
Diagnosis/Testing
No formal diagnostic criteria have been developed for the testing of hypophosphatasia. It is useful to note, however, that all forms of this disorder (except pseudohypophosphatasia) demonstrate reduced activity of unfractionated serum alkaline phosphatase (ALP) and the presence of either one or two pathogenic mutations in ALPL.3 The clinical presentation alone can lead to a misdiagnosis of other skeletal disease; therefore, the ALP concentrations are useful in distinguishing this condition.15
Management
There are two aspects to the management of individuals with hypophosphatasia: supportive therapy and medical therapy. Both require input from a multidisciplinary team of healthcare professionals.
Supportive therapy may include one or more of the following:
Management of Hypercalcemia: This can be achieved with adequate hydration, restriction of dietary calcium and vitamin D, and in some cases thiazide diuretics and glucocorticoids.13,16
Respiratory Insufficiency: Ventilatory support is often provided for months in severely affected infants, with some of these patients requiring a tracheostomy.13 In severe cases, however, mechanical ventilation may be challenging due to thoracic deformity, muscle weakness, gracile ribs, fractures, tracheomalacia, and even pulmonary hypoplasia.16 Patients requiring tracheostomy may develop problems with speech and language as well as the ability to tolerate oral feeding. In such patients, speech therapists and dieticians may be required to step in and provide input.13
Seizures: Pyridoxine may be used for vitamin B6–dependent seizures; however, they may eventually become refractory to therapy.16
Craniosynostosis: A craniotomy may be performed on patients with neurological complications from “functional” or bony craniosynostosis.16
Dental Care: Since orodental symptoms are a prominent feature in patients with hypophosphatasia, and the premature loss of many teeth can impair speech and nutrition, dentists should closely monitor all individuals with hypophosphatasia.3,6,17
Scoliosis: Severely affected individuals with scoliosis may require surgery.
Pain: Many patients complain of pain, and many have some degree of motor difficulty. Such patients may need physiotherapy, occupational therapy, and the input of a chronic pain management team; in some cases, naproxen may be appropriate.13,16
Fractures: Fractures may heal with difficulty, and femoral or pseudofractures may require load-sharing intramedullary fixation.13,16 Metatarsal fractures may heal better with ankle/foot orthoses.16
Physical Therapy: Physical therapy in children and infants may prove beneficial; however, physical therapists may lack education regarding the disease, and physical therapy may be underutilized.18 Therapists need to have a better understanding of the disease characteristics as well as when and how to intervene in order to optimally impact body function, lessen structural impairment, and facilitate increased functional independence in mobility and activities of daily living. With this information, therapists will be better prepared to individualize treatment and achieve better outcomes.18
Medical Treatment
Conventional therapies for rickets or osteomalacia are best avoided unless deficiencies are identified, as excesses could worsen hypercalcemia or hypercalciuria.16 Bisphosphonates that are chemical analogues of one of the mineralization inhibitors, pyrophosphate, that accumulate in hypophosphatasia, are contraindicated; they are likely to worsen clinical outcomes. It is equally important to rule out hypophosphatasia when starting bisphosphonate therapy in older patients with a low ALP at presentation.13
Bone marrow and stem-cell transplantation in infancy and childhood have rescued two girls dying from infantile hypophosphatasia and ameliorated the severity of the disease, but these modalities failed to provide long-term improvement in bone structure.19,20
Teriparatide (parathyroid fragment 1-34) has shown some benefit for adult hypophosphatasia, with documented healing of a femoral pseudofracture and metatarsal stress fractures.21 More recently, parathyroid hormone was successfully used to aid fracture healing.22
Until 2015, however, there had not been any major successful medical therapy for the management of hypophosphatasia, and treatment had been primarily supportive. Asfotase alfa, marketed as Strensiq by Alexion Pharmaceuticals in the United States, is a bone-targeted, human TNSALP developed to treat hypophosphatasia.14 It was approved by the FDA in 2015 and is the first therapy for hypophosphatasia.23 Asfotase alfa is a soluble glycoprotein composed of two identical polypeptide chains, each with 726 amino acids. It is created with recombinant DNA technology in Chinese hamster ovary cells and is currently indicated for the treatment of patients with perinatal/infantile- and juvenile-onset hypophosphatasia.23,24
Asfotase alfa has been associated when skeletal, respiratory, and functional improvement in patients with perinatal/infantile- and juvenile-onset hypophosphatasia treated for up to 7 years. Comparative trials with age-matched historical controls showed that bone mineralization, survival, and ventilation-free survival substantially improved in patients with perinatal (severe) and infantile hypophosphatasia. Those patients with childhood hypophosphatasia demonstrated improved growth, gross motor function, strength and agility, and decreased pain.25
Strensiq is available as a solution for SC injection in single-use vials of 18 mg/0.45 mL, 28 mg/0.7 mL, 40 mg/mL, or 80 mg/0.8 mL.23 The 80-mg/0.8-mL vials should not be used in pediatric patients under 40 kg. The vials must be stored between 2°C and 8°C, protected from light, and must not be frozen or shaken. The recommended dose is 2 mg/kg SC three times per week or 1 mg/kg SC six times per week. In cases of insufficient efficacy in perinatal or infantile onset, the dose may be increased to 3 mg/kg three times per week.23
Injection-site reactions are the most common adverse effects in asfostase alfa trials, occurring in more than 60% of patients. However, most injection-site reactions resolved within a week, and the compound is generally well tolerated. Other adverse events, including lipodystrophy, hypersensitivity reactions, and ectopic calcifications in the eyes and kidneys, are of mild-to-moderate intensity.23,25 Patients should have ophthalmologic examinations to monitor for ectopic calcifications in the eyes and renal ultrasounds to monitor the kidneys.23
Role of the Pharmacist
Pharmacists form a vital part of the multidiscplinary team that manages patients with hypophosphatasia, providing their input at various stages of the condition. Some of the key points pharmacists can make include23:
• Ensuring that elderly patients who take bisphosphonates have not been misdiagnosed and do not suffer from hypophosphatasia
• Guiding patients who present with possible symptoms of hypophosphatasia on how to seek medical care and advice
• Stressing the importance of genetic counseling to all families that have affected children and suggesting prenatal testing for the perinatal form to parents
• Providing adequate counseling when dispensing asfotase alfa for home use. See Table 2 for some of the key points that pharmacists can discuss with patients.

Conclusion
Pharmacists can assist with the diagnosis and management of patients with hypophosphatasia. Even though the management of the disease is not well established, there is now a medication approved for the treatment of hypophosphotasia. In addition to guiding patients on supportive therapy, pharmacists can ensure the correct use of any prescribed medication.
REFERENCES
1. Tenorio J, Alvarez I, Riancho-Zarrabeitia L, et al. Molecular and clinical analysis of ALPL in a cohort of patients with suspicion of Hypophosphatasia. Am J Med Genet A. 2017;173(3):601-610. Epub January 27, 2017.
2. Mornet E. Hypophosphatasia. Orphanet J Rare Dis. 2007;2(1):40.
3. Plotkin H. Hypophosphatasia. Medscape. Updated December 11, 2015. http://emedicine.medscape.com/article/945375-overview#showall. Accessed January 17, 2017.
4. Mornet E, Nunes ME. Hypophosphatasia. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews. Seattle, WA: University of Washington, Seattle. 1993-2017.
5. Fraser D. Hypophosphatasia. Am J Med. 1957;22(5)730-746.
6. Greenberg CR, Evans JA, McKendry-Smith S, et al. Infantile hypophosphatasia: localization within chromosome region 1p36.1-34 and prenatal diagnosis using linked DNA markers. Am J Hum Genet. 1990;46(2)286-292.
7. Greenberg CR, Taylor CL, Haworth JC, et al. A homoallelic Gly317-->Asp mutation in ALPL causes the perinatal (lethal) form of hypophosphatasia in Canadian mennonites. Genomics. 1993;17(1)215-217.
8. Orton NC, Innes AM, Chudley AE, Bech-Hansen NT. Unique disease heritage of the Dutch-German Mennonite population. Am J Med Genet A. 2008;146a(8):1072-1087.
9. Watanabe A, Karasugi T, Sawai H, et al. Prevalence of c.1559delT in ALPL, a common mutation resulting in the perinatal (lethal) form of hypophosphatasia in Japanese and effects of the mutation on heterozygous carriers. J Hum Genet. 2011;56(2):166-168.
10. Whyte MP, Essmyer K, Geimer M, Mumm S. Homozygosity for TNSALP mutation 1348c>T (Arg433Cys) causes infantile hypophosphatasia manifesting transient disease correction and variably lethal outcome in a kindred of black ancestry. J Pediatr. 2006;148(6):753-758.
11. Mornet E, Yvard A, Taillandier A, et al. A molecular-based estimation of the prevalence of hypophosphatasia in the European population. Ann Hum Gen. 2011;75(3):439-445.
12. Weber TJ, Sawyer EK, Moseley S, et al. Burden of disease in adult patients with hypophosphatasia: results from two patient-reported surveys. Metabolism. 2016;65(10):1522-1530.
13. Bishop N. Clinical management of hypophosphatasia. Clin Cases in Miner Bone Metab. 2015;12(2):170-173.
14. Whyte MP, Madson KL, Phillips D, Reeves AL, et al. Asfotase alfa therapy for children with hypophosphatasia. JCI Insight. 2016;1(9):e85971.
15. Rockman-Greenberg C. Hypophosphatasia. Pediatr Endocrinol Rev. 2013;10 (suppl 2):380-388.
16. Whyte MP. Hypophosphatasia: enzyme replacement therapy brings new opportunities and new challenges. J Bone Miner Res. 2017; January 2013. Epub ahead of print.
17. Bloch-Zupan A. Hypophosphatasia: diagnosis and clinical signs—a dental surgeon perspective. Int J of Paediatr Dent. 2016;26(6):426-438.
18. Phillips D, Case LE, Griffin D, et al. Physical therapy management of infants and children with hypophosphatasia. Mol Genet and Metab.119(1):14-19.
19. Whyte MP, Kurtzberg J, McAlister WH, et al. Marrow cell transplantation for infantile hypophosphatasia. J Bone Miner Res. 2003;18:624-636.
20. Cahill RA, Wenkert D, Perlman SA, et al. Infantile hypophosphatasia: transplantation therapy trial using bone fragments and cultured osteoblasts. J Clin Endocrinol Metab. 2007;92(8):2923-2930.
21. Whyte MP, Mumm S, Deal C. Adult hypophosphatasia treated with teriparatide. J Clin Endocrinol Metab. 2007;92(4):1203-1208.
22. Camacho PM, Mazhari AM, Wilczynski C, et al. Adult hypophosphatasia treated with teriparatide: report of 2 patients and review of the literature. Endocr Pract. 2016;22(8):941-950.
23. Strensiq [package insert] New Haven, CT: Alexion Pharmaceuticals; 2016. www.strensiq.com/images/Strensiq_PRESCRIBING_INFORMATION.pdf.
24. Morrow T. Expensive new biologic helps children fight hypophosphatasia. Manag Care. 2015;24(12):25-26.
25. Hofmann C, Seefried L, Jakob F. Asfotase alfa: enzyme replacement for the treatment of bone disease in hypophosphatasia. Drugs Today. 2016;52(5):271-285.
To comment on this article, contact rdavidson@uspharmacist.com.






