US Pharm. 2006;12:HS8-HS30.
Kidney, heart, liver, and lung transplantations have become relatively frequent clinical options for diseases with irreversible consequences. One-year graft survival rates for kidney, heart, and liver transplantations are reportedly between 84% and 98%. However, advances in surgical techniques of organ transplantation produce new challenges in promoting organ acceptance.
The transplant of an organ or tissue from one individual to another of the same species with a different genotype is called an allograft. Allografts account for many of the human transplants, including those from cadavers, living-related, and living-unrelated donors. In 2004, a total of 27,036 organ transplants were performed in the United States. A majority (20,045 transplants) were from deceased organ donors, while 6,991 were from living donors.1
Due to the complexity of the immune reaction, an in-depth review is beyond the scope of this article; yet, the reader should note that the most important mediator of the immune response includes the T cell lymphocytes, and the most salient cell messengers include interleukins (ILs), particularly IL-1 and IL-2. Graft rejection follows a sequence of events that involves detection of donor histo compatability (the process involved with molecules that are displayed on cell surfaces responsible for lymphocyte recognition and antigen presentation) differences by the recipient's immune system, enrollment of activated lymphocytes, activation of immune effector mechanisms, and ultimately, rejection of the graft. Grafts can be rejected within minutes of transplantation when donor-specific antibodies are present at the time of the transplant (hyperacute rejection). Rejection can be mediated by alloreactive T lymphocytes that infiltrate the allograft (acute cellular rejection). Perhaps the most important source of rejection gradually occurs years after the initial transplant and involves loss of graft function (chronic rejection).
Since organ transplantation is now a feasible option for many patients with end-stage organ failure, an ever-increasing number of immunosuppressive agents targeting specific steps of the immunologic response to the transplanted organ is available. This article presents an overview of the fundamental use of and differences between pharmacologic methods of immunosuppression, as well as the advantages of the newer medications. Ultimately, this information has implications for pharmacists and how they contribute to better patient outcomes.
Corticosteroids
Corticosteroids have been used as
immunosuppressive drugs in transplantation for several years and are
considered the first-line drug treatment of acute allograft rejection.
Prednisone and prednisolone are commonly used as part of the prophylactic
regimen in immunosuppression, while methylprednisolone is used to treat acute
rejection episodes. Corticosteroids bind to glucocorticoid receptors located
in the cell cytoplasm and inhibit production of various proinflammatory and
immunomodulatory cytokines, such as IL-1, IL-2, IL-6, and tumor necrosis
factor-alpha. They also impair activity of B cells, T cells, and macrophages.
Dosage of corticosteroids varies depending on their use as initiation or maintenance therapy as well as the individual's tolerability to the drug. Prednisone is metabolized to prednisolone in the liver by the cytochrome P450 3A (CYP3A) enzymes, and both drugs may require dose reduction in severe hepatic impairment. Methylprednisolone is also metabolized by the same cytochrome enzyme system and can be administered either by injection or by mouth. Methylprednisolone sodium succinate (Solu-Medrol) can be given either intravenously (IV) or intramuscularly (IM). Due to its higher solubility, it has a more rapid onset of action than methylprednisolone acetate.2,3
Corticosteroids are known to cause a variety of adverse effects. Some reactions of particular importance in the transplant population include hyperglycemia, diabetes mellitus, edema, hypertension, hyperlipidemia, hypokalemia, hirsutism, arthralgia, osteoporosis, and psychosis. Moreover, since a large majority of patients are maintained on oral steroid therapy for months to years, it is important to remember several counseling points associated with chronic use of steroids. Prednisone itself may interfere with the absorption of calcium; thus, patients may need to increase their calcium intake. To decrease the risk of gastrointestinal (GI) bleeding and ulceration, steroids should be taken with food. Concomitant administration of steroids and nonsteroidal anti-inflammatory drugs (NSAIDs) may increase risk of GI bleeding as well.2-4
Studies have examined the risks and benefits of early steroid withdrawal--starting the steroid taper within the first three months posttransplant. For example, one prospective randomized, double-blind study conducted among 266 kidney transplant recipients receiving prednisone, cyclosporine, and mycophenolate mofetil indicated that early steroid withdrawal at three months was associated with less hypertension and hypercholesterolemia but a higher incidence of acute rejection and increased serum creatinine levels.5 Other studies that extended the withdrawal time over six to 12 months had smaller risks of acute rejection episodes. On the other hand, steroid withdrawal in liver transplant patients can be safely initiated at three months posttransplantation, assuming patients remain rejection-free during that time period.6
Steroid-free regimens have also been examined in renal transplant recipients. In an open-label, parallel-group, multicenter study, 451 renal transplant recipients were randomized to receive one of two steroid-free regimens (tacrolimus/mycophenolate mofetil or tacrolimus/basiliximab) or a standard triple regimen (prednisone/tacrolimus/mycophenolate mofetil). After six months, the steroid-free regimens had significantly higher rates of acute rejection, but patient and graft survival rates were similar among all groups. However, steroid-free regimens carry a relatively high risk of an acute rejection episode of about 25% to 30% in renal transplant recipients. Moreover, use of steroids is still necessary for transplantation of organs other than the kidney.7
Calcineurin Inhibitors
Cyclosporine and tacrolimus
(FK-506, Prograf) belong to the group of immunosuppressive agents called
calcineurin inhibitors(CNIs). The mechanism of action of the two drugs is
similar, as both bind to their own immunophilin molecule (cyclosporine to
cyclophilin and tacrolimus to FK506-binding protein 12). The complex of the
CNI with its respective binding protein inhibits activity of calcineurin--an
enzyme necessary for the induction of T cell activation and proliferation. As
a result of this interaction, T cell activation and proliferation is blocked,
and production of proinflammatory cytokines is inhibited.7
Several studies have shown that tacrolimus and cyclosporine have equivalent patient and graft survival rates posttransplantation.8,9 However, tacrolimus seems to have a lower incidence of acute rejection. One study evaluated 560 renal transplant patients who received either tacrolimus or cyclosporine microemulsion in combination with steroids and azathioprine. After six months posttransplantion, the group receiving tacrolimus had a 19.6% incidence of biopsy-proven acute rejection, compared to a 37.3% incidence for the cyclosporine group.8
Cyclosporine: Cyclosporine has been successfully used in transplantation, particularly renal, since the 1980s. Its use is approved in patients suffering from autoimmune conditions, rheumatoid arthritis, and psoriasis. Sandimmune was the original cyclosporine formulation, but its erratic absorption and highly variable inter- and intrapatient bioavailability made therapeutic monitoring difficult in some patients. This led to the development of cyclosporine microemulsion, or Neoral, which has a more consistent and reliable bioavailability profile. Sandimmune and Neoral are not interchangeable, and patients should not be switched from one formulation to the other without close monitoring.10,11
Even though cyclosporine blood monitoring is necessary to ensure efficacy and avoid toxicity, there is no clear consensus on what levels are desirable. For example, cyclosporine levels vary depending on the type of organ transplanted, concomitant use of other immunosuppressants, and the analytical assay. In addition, clinicians need to individualize the dose due to patient variability in cyclosporine absorption and metabolism as well as tolerability and side effects. In contrast to other immunosuppressants, cyclosporine monitoring may consist of either trough or two-hour concentration (C2) monitoring. Therapeutic monitoring of cyclosporine two-hour postdose concentration seems to be especially beneficial in decreasing risk of acute rejection and nephrotoxicity during the first months of cyclosporine therapy.12 However, the benefit of C2 monitoring in chronic maintenance therapy is yet to be established.
Both oral Sandimmune and Neoral come in soft gelatin capsule (25 and 100 mg) and oral solution (100 mg/mL) formulations. Sandimmune injection is also available as a 50 mg/5 mL ampule. While the soft gelatin capsules and oral solution doses of the same brand are equivalent, Sandimmune IV to oral switch is 1 to 3, respectively. All cyclosporine products are taken twice daily, consistently at the same time.10,11
Cyclosporine is principally metabolized by the CYP3A4 enzyme system, and its bioavailability may be affected by substrates, inhibitors, and inducers of the same enzyme. Cyclosporine interactions with other immunosuppressants are particularly significant and may require dose adjustment. For example, corticosteroids may increase the concentration of cyclosporine. Cyclosporine itself increases the concentration of sirolimus by 2.3-fold. Also, cyclosporine may reduce clearance of digoxin, statins, colchicine, and prednisolone and may lead to toxicities of these drugs.11,13
Patients taking CNIs should be strongly urged to avoid use of some common products and consult their pharmacist or doctor before starting any herbal supplement. For example, there have been several reports of interactions between CNIs and St. John's Wort, a potent CYP3A inducer. This interaction can lead to decreased cyclosporine and tacrolimus levels in blood and subsequent transplant loss. Additionally, grapefruit juice inhibits the CYP3A enzyme system and may elevate cyclosporine concentration. Potassium-sparing diuretics may exacerbate hyperkalemia associated with CNIs, and coadministration with NSAIDs can elevate the potential for nephrotoxicity. Thus, these agents should be avoided in cyclosporine-treated patients as well. 10,11,14
The main side effects of CNIs are nephrotoxicity and neurotoxicity. Nephrotoxicity is usually reversible upon discontinuation of CNIs, but irreversible interstitial fibrosis and arteriolar changes in the kidney are observed with long-term use. Side effects associated with neurotoxicity include symptoms such as headache, tremor, and insomnia. Convulsions and psychosis are much less common but are more likely to occur if a patient has hypomagnesemia.15
Cyclosporine is less neurotoxic and diabetogenic than tacrolimus, although cyclosporine is associated with a higher incidence of hypertension, dyslipidemia, gingival hyperplasia, and hirsutism.15 Furthermore, the nephrotoxic potential of cyclosporine is an adverse reaction that has led to a gradual reduction of its use in the transplant population. Other common side effects associated with both tacrolimus and cyclosporine include hematologic, GI, and electrolyte abnormalities. Clinicians should be particularly aware of hyperkalemia and hypomagnesemia associated with CNIs. Patients will require frequent monitoring of serum electrolytes with a complete blood count, renal and hepatic function, glucose, blood pressure, and lipids.11,16,17
Tacrolimus: Tacrolimus was originally derived from the yeast species Streptomyces tsukubaensis. It is FDA approved for the prophylaxis of organ rejection in kidney, heart, and liver transplant recipients but is commonly used in transplantation of other organs as well. Tacrolimus comes in capsule (0.5, 1, and 5 mg) and IV 5-mg/mL formulations. Due to reported anaphylactic reactions with tacrolimus infusion, the IV route should be used only at the lowest dose possible for the shortest amount of time. When the patient is switched from IV to oral tacrolimus, the dose needs to be increased by about threefold. The topical formulation of tacrolimus (Protopic) and its derivative pimecrolimus (Elidel) are also used to treat autoimmune diseases such as eczema.16
Tacrolimus has a narrow therapeutic range that requires regular monitoring to guide dosing. Tacrolimus dosing therefore needs to be individualized and depends on the type of organ transplant received, immunosuppressive protocols, and analytical method used to measure tacrolimus trough concentration. Moreover, each patient needs to be frequently monitored for signs of excessive immunosuppression and toxicity, since individual tolerability to this drug varies widely from patient to patient. The manufacturer offers information on recommended trough concentration ranges in kidney and liver transplant recipients.16
Like cyclosporine, tacrolimus is metabolized by the CYP3A4 enzyme system and thus may interact with drugs that are substrates, inhibitors, or inducers of the same enzyme group. Most notable drug interactions that would necessitate a change in tacrolimus dose are associated with azole antifungals, macrolide antibiotics, anticonvulsants, and calcium channel blockers.16 Tacrolimus and sirolimus coadministration was found to be safe, without resulting in additional toxicity.18 In addition, coadministration of tacrolimus and mycophenolic acid (MPA) does not affect tacrolimus concentration, but some evidence shows that tacrolimus may increase MPA concentration.19 Tacrolimus and cyclosporine should not be used together because they have similar mechanisms of action that would not result in effective immunosuppression.11,16 Patients should avoid grapefruit juice, herbal supplements (e.g., St. John's Wort), and potassium-sparing diuretics because of the same concerns mentioned earlier in the cyclosporine section.11,16
Due to the extensive hepatic metabolism of tacrolimus by CYP3A4, dose reduction in moderate-to-severe hepatic impairment is necessary. The pharmacokinetics of tacrolimus will not be affected by renal impairment, although tacrolimus-associated nephrotoxicity may still warrant a dose reduction. Patients should be advised to take tacrolimus on an empty stomach, since the presence of food can decrease absorption of tacrolimus by almost one third.11, 16
Nephrotoxic and neurotoxic side effects of tacrolimus are similar to those of cyclosporine; however, tacrolimus is associated with greater tremor. Forty percent of liver transplant and 52% of kidney transplant patients experienced nephrotoxicity while on tacrolimus therapy. Side effects associated with neurotoxicity are present in about 55% of tacrolimus-treated liver transplant patients. Another side effect that is significantly more prevalent with tacrolimus therapy is posttransplant diabetes mellitus16,17 (PTDM). A meta-analysis from 1992 to 2002 found that incidence of insulin-dependent PTDM was almost four times higher in tacrolimus-treated patients than in cyclosporine-treated patients (9.8% vs. 2.7%).20 The risk of developing PTDM is greatest during first-year posttransplant, with reportedly 19.9% of patients requiring insulin therapy. However, between first- and fifth-year posttransplant, the incidence of PTDM decreases to only 2.6%.9,21 Since the diabetogenic effect is limited only to decreased insulin production from beta pancreatic cells, it seems to be reversible in over 40% of patients after five years of tacrolimus therapy.21
mTOR Inhibitors
Sirolimus:
Mammalian target of rapamycin (mTOR) inhibitors are a relatively new drug
class of immunosuppressants and consist of two agents: sirolimus and
everolimus. They inhibit T cell proliferation by first binding to immunophilin
FKBP12 and then inhibiting mTOR, a key regulatory kinase involved in cell
cycle progression and proliferation.22 Sirolimus (Rapamune) is a
macrolide antibiotic originally derived as a yeast product from
Streptomyces hygroscopicus and is approved by the FDA for
immunosuppressive prophylaxis in combination with cyclosporine and
corticosteroids in renal transplant recipients. (Cyclosporine may be withdrawn
in low-to-moderate immunological risk patients after two to four months, in
conjunction with an increase in sirolimus dosage.) In addition,
sirolimus-eluting coronary stents (Cypher) have been used successfully to
reduce the risk of restenosis after coronary angiography.23,24
Sirolimus has been extensively studied in renal transplantation. A few studies have also been conducted in liver transplantation, but its use has been limited by the association with hepatic artery thrombosis.17,25 Sirolimus is a highly effective immunosuppressant, and initial studies showed significant reductions in rejection incidence. The Rapamune Global Study Group, a randomized, double-blind, placebo-controlled trial, conducted a multicenter clinical study among 576 renal transplant recipients on concomitant cyclosporine and steroid therapy. Patients were randomized to receive either 2 or 5 mg/day of sirolimus or placebo. After six months, the incidence of biopsy-proven rejection was 19%, 11%, and 37%, respectively.26 Recent clinical trials have reported success regarding the use of sirolimus to allow withdrawal from cyclosporine therapy and thus reduce risk of nephrotoxicity associated with the latter agent.
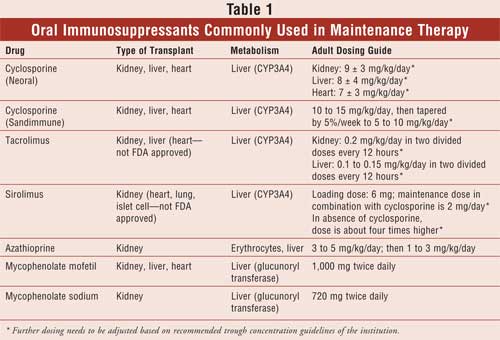
Sirolimus is available as 1- and 2-mg tablets, as well as a 1-mg/mL oral solution. The oral solution and the tablet formulation have been shown to be equivalent and can be interchanged on a milligram-for-milligram basis. In de novo kidney transplant recipients, initiation of sirolimus requires administration of a loading dose that is three times the maintenance dose (see Table 1 for dosage guidelines). Sirolimus dosing should be guided by its trough concentration, since there can be significant differences in patient-to-patient pharmacokinetics, with dose-adjusted bioavailability varying by about 50%. Sirolimus also has a narrow therapeutic range; thus, its trough concentration should be monitored. The target therapeutic range of sirolimus varies depending on whether the patient is receiving concomitant calcineurin inhibitor therapy. It is important to remember that dose adjustment of sirolimus should not be based solely on trough concentration alone but should also take into account clinical signs and symptoms, laboratory values, and tissue biopsy.25
Sirolimus is primarily metabolized by CYP3A4 and thus may interact with numerous drugs that are metabolized by the same enzyme system. Monitoring for drug interactions becomes crucial in patients on sirolimus because of its narrow therapeutic range. Significant changes in concentration may result in either transplant rejection or toxicity. Inhibitors of CYP3A4, such as azole antifungals and diltiazem, may significantly increase the concentration of sirolimus, while inducers of CYP3A4, such as rifampin and phenytoin, may decrease it.26 One of the most important sirolimus interactions is associated with cyclosporine, since the two drugs are often prescribed together. A study conducted among 24 healthy volunteers showed that sirolimus concentration increased 2.3-fold when it was administered at the same time with cyclo sporine (Neoral), although cyclosporine levels were not affected. As a result, these two medications should be taken at least four hours apart to decrease the substantial changes in sirolimus concentration. Since sirolimus bioavailability is 35% higher in the presence of a high-fat meal than it is under fasting conditions, patients also need to be advised to consistently take sirolimus at the same time either before or after a meal. While no dosage adjustment is necessary in renal impairment, sirolimus dose needs to be decreased by one third in patients with hepatic impairment.25
Main adverse effects of sirolimus are dose-dependent and include dyslipidemia, hypertension, thrombocytopenia, anemia, peripheral edema, and tremor. Dyslipidemia, specifically elevated cholesterol and triglyceride levels, can be particularly troublesome due to the high prevalence (as least 60%) in renal transplant population and the association with chronic graft failure. Rashes, acne, and mouth ulcerations are also more common in patients taking mTOR inhibitors. Bone marrow suppression is mostly a problem during the first months of therapy but is probably due to a combination of the immunosuppressive regimen with high-dose steroids.17,25
Overall, sirolimus is an effective immunosuppressive drug that represents a valuable alternative for transplant patients that avoids nephrotoxic effects of cyclosporine. Since sirolimus is mainly metabolized by CYP3A4 enzymes, it does have the potential for significant drug interactions with other drugs affecting the same enzyme group. Besides measuring sirolimus levels, lipid panel, blood pressure, and blood elements will need to be monitored as well. Additionally, patients using sirolimus should be advised to avoid grapefruit juice, herbal supplements, and talk to their provider or pharmacist first before taking any OTC medications. 17,25
Everolimus (Certican) is a derivative of sirolimus with a similar mechanism of action and a comparable side-effect profile to sirolimus. It was initially formulated to have a better absorption profile from the GI tract and thus improved bioavailability in the body, compared to sirolimus.27 Everolimus is still being evaluated in clinical trials and is pending FDA approval.
Antiproliferative Agents
Azathioprine:
Azathioprine (Imuran) was first used for transplantation in the early 1960s,
initially in combination with steroids and later as part of the triple therapy
that included steroids and cyclosporine. Currently, azathioprine use has
significantly declined, as it is being replaced by MPA, a more specific purine
inhibitor. Azathioprine is commercially available in oral (50-mg tablet) and
IV (100 mg/20 mL) formulations that are dose equivalent to each other28
(see Table 1 for dosing guidelines).
Azathioprine is metabolized to 6-mercaptopurine (6-MP), which is either inactivated by two enzymes, thiopurine s-methyltransferase (TPMT) and xanthine oxidase (XO), or is further metabolized to thioinosine monophosphate (TIMP). TIMP then undergoes more enzymatic modification to inhibit de novo purine synthesis. Of note, a common genetic polymorphism involving the inactivating enzyme TPMT can alter this metabolic pathway and lead to dangerously high levels of the active metabolite of azathioprine. In fact, 10% of Caucasians and African-Americans are heterozygous for the inactive TPMT allele, and 0.3% are homozygous for both inactive alleles. Patients with impaired TPMT metabolism are at especially high risk of experiencing myelosuppression associated with azathioprine toxicity. Another contributing factor that may lead to elevated azathioprine levels is concomitant allopurinol administration. Allopurinol inhibits XO, the other enzyme involved in degradation of 6-MP, and thus may elevate azathioprine bioavailability by fivefold. It is generally not recommended to administer azathioprine and allopurinol together, but if necessary, the dose of azathioprine should be reduced to one third to one fourth of the usual recommended dose.17,28
The main side effect of azathioprine is dose-dependent bone marrow suppression that may occur in over half of treated patients. Hepatotoxicity may occur in 2% to 10% of renal transplant recipients receiving azathioprine, necessitating regular monitoring of liver function tests. GI symptoms, mostly nausea and vomiting, are common during the first months of therapy. The azathioprine dose requires adjustment in renal impairment. The dose should be reduced by 25% if the creatinine clearance (CrCl) is below 50 mL/minute. Only 50% of the normal dose should be given to patients with CrCl below 10 mL/minute.17, 28
In contrast to the other immunosuppressants, azathioprine concentration is not routinely monitored in transplant recipients. For example, one prospective, randomized study evaluated the benefit of monitoring azathioprine metabolites in red blood cells.29 This study reported that the group of patients monitored for azathioprine metabolites had a lower incidence of acute rejection by 21% after three months posttransplant, compared to the group not receiving any azathioprine monitoring. However, there was no significant difference in graft survival between the two groups after six months. Currently, there is still no clear long-term evidence that measuring azathioprine metabolites in red blood cells actually correlates with therapeutic efficacy and toxicity.17,28
Overall, azathioprine is an effective immunosuppressant that has been used for years in combination with steroids and cyclosporine. However, the high incidence of myelosuppression and introduction of newer and more effective immunosuppressants have significantly reduced its use in transplantation.
Mycophenolic Acid: MPA is the active compound of two commercially available drugs, mycophenolate mofetil (MMF, CellCept) and a new delayed-release formulation, mycophenolate sodium (MPS, Myfortic). The main target of MPA is inosine monophosphate dehydrogenase (IMPDH), an enzyme essential for the de novosynthesis of guanosine nucleotides. Most cell types produce guanosine nucleotides by either the IMPDH involvement or a second salvage pathway. However, T and B lymphocytes lack the salvage pathway, so that the IMPDH inhibition by MPA ultimately leads to relatively selective suppression of lymphocyte proliferation.30,31
The pharmacokinetics of MPA are somewhat more complex than those of other immunosuppressants. Both MMF and MPS are quickly metabolized to its active drug, MPA, which then undergoes hepatic metabolism to its pharmacologically inactive metabolite MPA glucuronide. The MPA glucuronide is then converted back to MPA by enterohepatic recirculation, which results in a second peak in the MPA concentration about six to 12 hours postdose.17,30-32
Studies have demonstrated that MMF was superior to azathioprine in reducing the incidence of acute rejection episodes in conjunction with corticosteroids and cyclosporine. For example, the International Mycophenolate Mofetil Renal Transplant Study Group conducted a pooled analysis of three randomized, double-blind, multicenter clinical trials among almost 1,500 patients.33 Two standardized MMF dosages (2 g/day and 3 g/day) were compared to placebo or azathioprine as adjuncts to cyclosporine and corticosteroids. The incidence of acute rejection episodes was 40.8% for the placebo/azathioprine group, 19.8% for the MMF 2 g/day group, and 16.5% for the MMF 3 g/day group. MMF-treated groups also had less severe rejection episodes, better graft function over 12 months, and similar graft survival to placebo/azathioprine-treated groups.
It is recommended to take MMF and MPS on an empty stomach. In addition, MPS should not be crushed, chewed, or cut in order to maintain the enteric coated formulation. Absorption of MPA is decreased in the presence of antacids and cholestyramine, and patients should avoid any drugs that interfere with enterohepatic recirculation. When it comes to interactions with other immunosuppressants, cyclosporine was found to decrease the concentration of MPA by interfering with the enterohepatic recirculation of the drug. The interaction between MPA and tacrolimus is not completely understood, but potentially, tacrolimus may increase MPA concentration by inhibiting metabolism of MMF. Also, steroids are thought to induce glucuronosyl transferase, the enzyme responsible for conversion of MPA to MPA glucuronide.30-32
One of the biggest advantages of MPA is its lack of nephrotoxicity. In fact, experimental animal models have even demonstrated synergistic activity with angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in the treatment of nephropathy.34 Furthermore, it does not cause hypertension and dyslipidemia, in contrast to mTOR inhibitors. MPA also has some anti-inflammatory properties by its inhibition of IL-1 activity. Most common side effects associated with MMF include diarrhea, vomiting, and leukopenia. There is also an increased risk of sepsis in renal transplant recipients that seems to be caused by the cytomegalovirus viremia in a majority of cases.30,31
MMF is available as a 250-mg capsule, 500-mg tablet, 200-mg/mL oral suspension, and 6-mg/mL IV solution. It is approved for the prophylaxis of organ rejection in renal, cardiac, and hepatic transplantation in combination with steroids and cyclosporine. The usual dose ranges from 2 to 3 g/day divided in two doses. MPS is FDA approved in renal transplant patients in combination with steroids and cyclosporine. MPS comes as 180- and 360-mg delayed-release capsules, with a standard dose of 720 mg twice daily.
In studies conducted by the manufacturer, 1 g of MMF was equivalent to about 720 mg of MPS.30,31 However, the manufacturer does not recommend switching patients from MMF to MPS without doctor supervision because of the difference in the rate of absorption between the two formulations. No dose adjustment is necessary in hepatic parenchymal disease, but no data yet exist to determine whether hepatic impairment due to other causes would require dose adjustment. Patients with severe renal impairment (glomerular filtration rate <25 mL/minute) should avoid doses greater than 2 g/day due to accumulation of MPA and its glucuronide metabolite in the body.30,31
Therapeutic drug monitoring of MPA is not widely implemented due to insufficient evidence that MPA monitoring will improve efficacy and decrease toxicity in patients. Several studies have found a correlation between MPA exposure and risk of acute rejection but the therapeutic window has not been clearly defined.17, 32 Currently, a large multicenter trial is evaluating the benefit of MPA monitoring by comparing fixed-dose MPA to adjustable MPA dosing based on a measured blood concentration range. The results of this study are expected to be available soon and will hopefully help determine the effectiveness of therapeutic monitoring of MPA.35
Antilymphocyte-Depleting Antibodies: Acute rejection during first-year posttransplant is a major predictor of graft survival after renal transplantation. Use of induction therapy prior to and during the first weeks of posttransplantation with an antilymphocyte-depleting antibody or an IL-2 receptor (IL-2R) antagonist can provide effective protection against rejection during those first critical weeks and months posttransplantion. Maintenance therapy, in contrast, involves continuous administration of immunosuppressive agents discussed earlier in this article that are used to prevent acute rejection.36-39
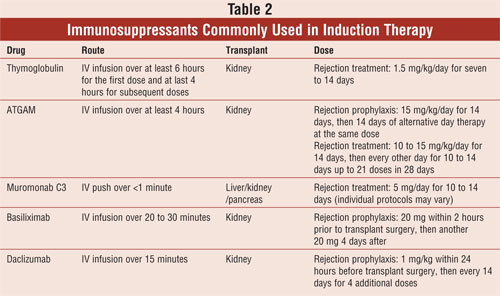
Polyclonal Antibodies: Polyclonal antilymphocyte-depleting antibodies, ATGAM and Thymoglobulin, are occasionally used in the early period after organ transplantation. The main purpose of their use is to reduce the risk of acute rejection, which itself is a strong predictor of graft loss. These antibodies are of either rabbit or equine origin and are directed against T and B lymphocytes, ultimately leading to T cell lysis and blockade of B cell activation. Even though there is a decreased risk of acute rejection, a retrospective data analysis from over 38,000 kidney transplant patients showed no improvement in graft survival or mortality with the use of these agents.36,37
The most common side effect associated with the use of polyclonal antibodies is the cytokine release syndrome that is present in up to 80% of patients. This reaction occurs after a patient receives the first dose and is characterized by fever, chills, diarrhea, dyspnea, and tachycardia. About half of patients also experience thrombocytopenia and leukopenia. Patients are usually premedicated with steroids, diphenhydramine, and acetaminophen to decrease the severity of the reaction. Also, an interdermal skin test containing 1:1,000 dilution of ATGAM in normal saline is recommended prior to the first ATGAM infusion to identify patients at risk for anaphylaxis. Thymoglobulin carries an increased risk of posttransplant lymphoproliferative disease (PTLD), a type of lymphoma with a mortality rate that can be as high as 50% to 80%.37
The tolerability and efficacy profiles differ quite significantly among the available polyclonal antibodies. Thymoglobulin and ATGAM were compared in a randomized, double-blind, multicenter trial, and after one year, Thymoglobulin was found to have significantly less rejection episodes than ATGAM (4% vs. 25%, respectively). Also, Thymoglobulin-treated patients had better event-free survival rates (defined as freedom from death, graft loss, and rejection) than ATGAM-treated patients. On the other hand, 56% of patients on Thymoglobulin experienced leukopenia, compared to only 4% of patients receiving ATGAM. Still, Thymoglobulin seems to be the preferred agent in clinical practice due to published data on its superior efficacy.38
Monoclonal Antibodies: Muromonab C3 (Orthoclone, OKT3) is a monoclonal antibody that may also be used in the treatment of acute rejection in transplant recipients. It binds to the CD3 molecule found on the surface of T cells, interferes with their antigen recognition, makes T cells immunologically inactive, and causes rapid T cell lysis. Similar to polyclonal antibodies, a majority of patients develop the cytokine release syndrome after the first doses of muromonab CD3, ranging in severity from mild flu-like symptoms to anaphylactic reactions. In addition, a registry analysis of over 38,000 kidney transplant recipients found that monoclonal antibodies increased risk of PTLD by 72% without having any significant effect on graft loss or mortality.36 The poor safety profile and adverse effects of muromonab C3 have limited its use in clinical practice to the treatment of steroid-resistant rejection, where it has been shown to be effective in approximately 87% of renal transplant recipients.37
IL-2R Antagonists
IL-2R antagonists basiliximab
(Simulect) and daclizumab (Zenopax) block IL-2–mediated activation of T cells
by binding to the CD25 subunit of the IL-2R. They have been studied mostly in
combination with CNIs, since CNIs block production of IL-2 and lead to a
synergistic blockade of IL-2 activity. Overall, IL-2R antagonists have been
found to be effective in reducing the incidence of acute rejection in renal
transplant patients.37 They are to be used only for the prophylaxis
and not for the treatment of acute rejection because during acute rejection, T
cells may become activated without the involvement of the CD25 subunit on the
IL-2 receptor. One randomized, double-blind study conducted among 380 renal
transplant patients treated with cyclosporine and steroids showed that the
addition of basiliximab significantly reduced the incidence of acute rejection
by 32% after six-month posttransplantation. Alternatively, there was only a
trend toward reduced incidence of graft loss (12.1% in the basiliximab group
vs. 13.4% in the placebo group, P = .591) that did not reach
significance.39
Retrospective analyses of basiliximab and daclizumab found the two IL-2R antagonists to be comparable in efficacy with similar effects on acute rejection and graft loss. The main difference lies in their binding affinity for the IL-2R and dosing schedule (see Table 2). Studies have demonstrated no increased risk of adverse effects with the use of IL-2R antagonists, compared to placebo. In addition, there is no increased risk of PTLD or cytokine release syndrome found with anti-lymphocyte-depleting antibodies. Although uncommon, there are reports of anaphylactic reactions to IL-2 antagonists.37,39 Both drugs are administered IV and carry no specific dosing recommendations for renally and/or hepatically impaired patients.
The Role of the Pharmacist
Pharmacists obviously have an
important role in improving outcomes for transplantation patients. Pharmacists
in the hospital may have the opportunity to make pharmacotherapeutic
recommendations that could increase the survivability of solid organ
transplant patients. For example, pharmacists can recommend sirolimus dose
reductions for patients with hepatic dysfunction. Certainly, community
pharmacists can have a vital role in educating the patient with regard to
medication adherence and self-monitoring for adverse affects. Likely more
important, once in the community, there are many drug and some food and herbal
interactions that may increase or decrease blood levels of immunosuppressants.
Without a doubt, the opportunity to counsel these patients presents an
important area of communication that could be life-saving. Furthermore,
diligently following-up with these patients on a regular basis may
significantly contribute to their long-term survival.
References
1. United Network for Organ
Sharing (UNOS)/Organ Procurement and Transplantation Network (OPTN). July 29,
2005.
2. Pfizer, Pharmacia & Upjohn
Company. Solu Medrol package insert. Kalamazoo, MI, October 2001.
3. Pfizer, Pharmacia & Upjohn
Company. Depo Medrol package insert. Kalamazoo, MI, March 2003.
4. Baciewicz AM, Baciewicz FA, Jr.
Cyclosporine pharmacokinetic drug interactions. Am J Surg.
1989;157:264-271.
5. Ahsan N, Hricik D, et al.
Prednisone withdrawal in kidney transplant recipients on cyclosporine and
mycophenolate mofetil--a prospective randomized study. Steroid Withdrawal Study
Group. Transplantation.1999;68:1865-1874.
6. Greig P, Lilly L, et al. Early
steroid withdrawal after liver transplantation: the Canadian tacrolimus versus
microemulsion cyclosporin A trial: 1-year follow-up. Liver Transpl.
2003; 9:587-595.
7. Vitko S, Klinger M, et al. Two
corticosteroid-free regimens-tacrolimus monotherapy after basiliximab
administration and tacrolimus/mycophenolate mofetil-in comparison with a
standard triple regimen in renal transplantation: results of the Atlas study.
Transplantation.2005;80:1734-1741.
8. Margreiter R. Efficacy and safety
of tacrolimus compared with ciclosporin microemulsion in renal
transplantation: a randomised multicentre study. Lancet.
2002;359:741-746.
9. Pirsch JD, Miller J, et al. A
comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after
cadaveric renal transplantation. FK506 Kidney Transplant Study Group.
Transplantation. 1997;63:977-983.
10. Novartis Pharmaceuticals.
Sandimmune package insert. East Hanover, NJ, August 2005.
11. Novartis Pharmaceuticals. Neoral
package insert. East Hanover, NJ, August 2005.
12. Levy G, Burra P, et al. Improved
clinical outcomes for liver transplant recipients using cyclosporine
monitoring based on 2-hr post-dose levels (C2). Transplantation.
2002;73:953-959.
13. Kaplan B, Meier-Kriesche HU, et
al. The effects of relative timing of sirolimus and cyclosporine microemulsion
formulation coadministration on the pharmacokinetics of each agent. Clin
Pharmacol Ther.1998;63:48-53.
14. Bauer S, Stormer E, et al.
Alterations in cyclosporin A pharmacokinetics and metabolism during treatment
with St John's wort in renal transplant patients. Br J Clin Pharmacol.
2003; 55:203-211.
15. Mayer AD, Dmitrewski J, et al.
Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in
the prevention of renal allograft rejection: a report of the European
Tacrolimus Multicenter Renal Study Group. Transplantation.
1997;64:436-443.
16. Astellas Pharma US, Inc. Prograf
package insert. Deerfield, IL, July 2005.
17. Taylor AL, Watson CJ, Bradley JA.
Immunosuppressive agents in solid organ transplantation: Mechanisms of action
and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56:23-46.
18. McAlister VC, Mahalati K, et al.
A clinical pharmacokinetic study of tacrolimus and sirolimus combination
immunosuppression comparing simultaneous to separated administration. Ther
Drug Monit.2002;24:346-350.
19. Christians U, Jacobsen W, et al.
Mechanisms of clinically relevant drug interactions associated with
tacrolimus. Clin Pharmacokinet. 2002;41:813-851.
20. Heisel O, Heisel R, et al. New
onset diabetes mellitus in patients receiving calcineurin inhibitors: a
systematic review and meta-analysis. Am J Transplant. 2004;4:583-595.
21. Vincenti F, Jensik SC, et al. A
long-term comparison of tacrolimus (FK506) and cyclosporine in kidney
transplantation: evidence for improved allograft survival at five years.
Transplantation.2002;73:775-782.
22. Sehgal SN. Sirolimus: its
discovery, biological properties, and mechanism of action. Transplant Proc.
2003;35(3 Suppl):7S-14S.
23. Klugherz BD, Llanos G, et al.
Twenty-eight-day efficacy and phamacokinetics of the sirolimus-eluting stent.
Coron Artery Dis. 2002;13:183-188.
24. Marks AR. Sirolimus for the
prevention of in-stent restenosis in a coronary artery. N Engl J Med.
2003;349:1307-1309.
25. Wyeth Pharmaceuticals. Rapamune
package insert. Philadelphia, PA, January 2006.
26. MacDonald AS. A worldwide, phase
III, randomized, controlled, safety and efficacy study of a
sirolimus/cyclosporine regimen for prevention of acute rejection in recipients
of primary mismatched renal allografts. Transplantation.
2001;71:271-280.
27. Nashan B. Review of the
proliferation inhibitor everolimus. Expert Opin Investig Drugs.
2002;11:1845-1857.
28. Prometheus Laboratories, Inc.
Imuran package insert. San Diego, CA, October 2005.
29. Bergan S, Rugstad HE, et al.
Monitored high-dose azathioprine treatment reduces acute rejection episodes
after renal transplantation. Transplantation. 1998;66:334-339.
30. Novartis Pharmaceuticals.
Myfortic package insert. East Hanover, NJ, February 2004.
31. Roche Pharmaceuticals. Cellcept
package insert. Nutley, NJ, October 2005.
32. Van Gelder T, Hilbrands LB, et
al. A randomized double-blind, multicenter plasma concentration controlled
study of the safety and efficacy of oral mycophenolate mofetil for the
prevention of acute rejection after kidney transplantation. Transplantation.
1999;68:261-266.
33. Halloran P, Mathew T, et al.
Mycophenolate mofetil in renal allograft recipients: a pooled efficacy
analysis of three randomized, double-blind, clinical studies in prevention of
rejection. The International Mycophenolate Mofetil Renal Transplant Study
Groups. Transplantation. 1997;63:39-47.
34. Allison AC, Eugui EM. Mechanisms
of action of mycophenolate mofetil in preventing acute and chronic allograft
rejection. Transplantation. 2005;80(2 Suppl):S181-S190.
35. Van Gelder T, Shaw LM. The
rationale for and limitations of therapeutic drug monitoring for mycophenolate
mofetil in transplantation. Transplantation. 2005;80(2 Suppl):S244-S253.
36. Cherikh WS, Kauffman HM, et al.
Association of the type of induction immunosuppression with posttransplant
lymphoproliferative disorder, graft survival, and patient survival after
primary kidney transplantation. Transplantation. 2003;76:1289-1293.
37. Nashan B. Antibody induction
therapy in renal transplant patients receiving calcineurin-inhibitor
immunosuppressive regimens: a comparative review. BioDrugs.
2005;19:39-46.
38. Brennan DC, Flavin K, et al. A
randomized, double-blinded comparison of Thymoglobulin versus Atgam for
induction immunosuppressive therapy in adult renal transplant recipients.
Transplantation. 1999;67:1011-1018.
39. Nashan B, Moore R, et al.
Randomised trial of basiliximab versus placebo for control of acute cellular
rejection in renal allograft recipients. CHIB 201 International Study Group.
Lancet.1997;350:1193-1198.
To comment on this article, contact editor@uspharmacist.com.






